DC (Dendritic cell)-CIK (Cytokine-induced killer cell) immune cell modified by both AFP (Alpha-fetoprotein) and HBsAg (Hepatitis B surface antigen) antigen genes as well as preparation method and application of the cell
A technology of gene modification and immune cells, which is applied in the direction of genetically modified cells, cells modified by introducing foreign genetic material, blood/immune system cells, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Construction of recombinant lentiviral expression system for AFP and HBsAg
[0049] 1. Construction of pHAGE-AFP-HBsAg recombinant plasmid:
[0050] According to the full-length CDS sequences of AFP and HBsAg registered in GeneBank, we designed the following 2 pairs of primers, which introduced BamH Ⅰ, Xho Ⅰ, Xho Ⅰ and Nhe Ⅰ restriction sites at P1, P2, P3 and P4 respectively:
[0051] P1: GGATTC ATGAAGTGGGTGGAATCAAT
[0052] P2: CTCGAG TTAAACTCCCAAAGCAGCA
[0053] P3: CTCGAG ATGGGAGGTTGGTCTTCC
[0054] P4: GCTAGC GTAGATCTAAGGCATCAAGGC
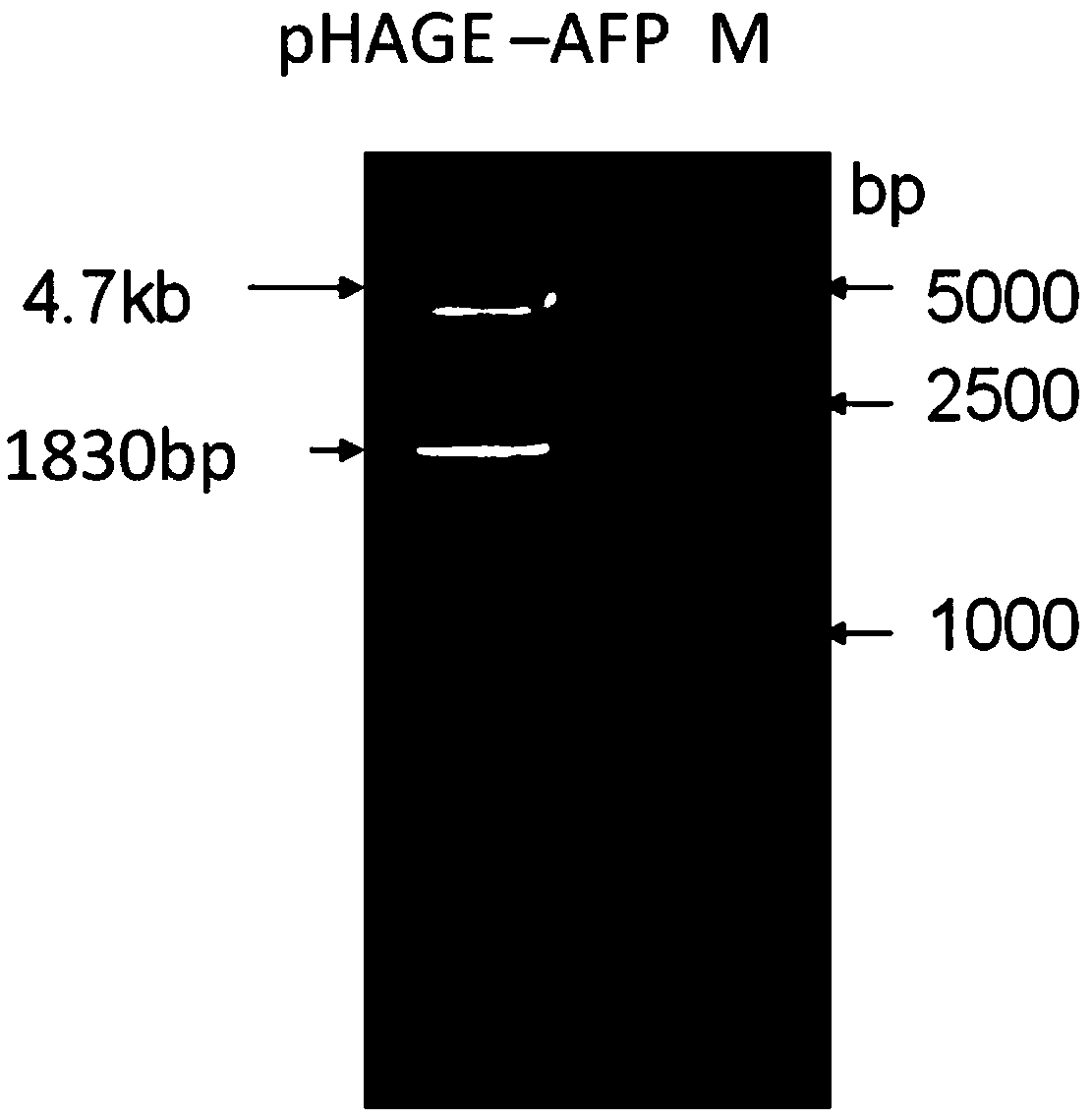
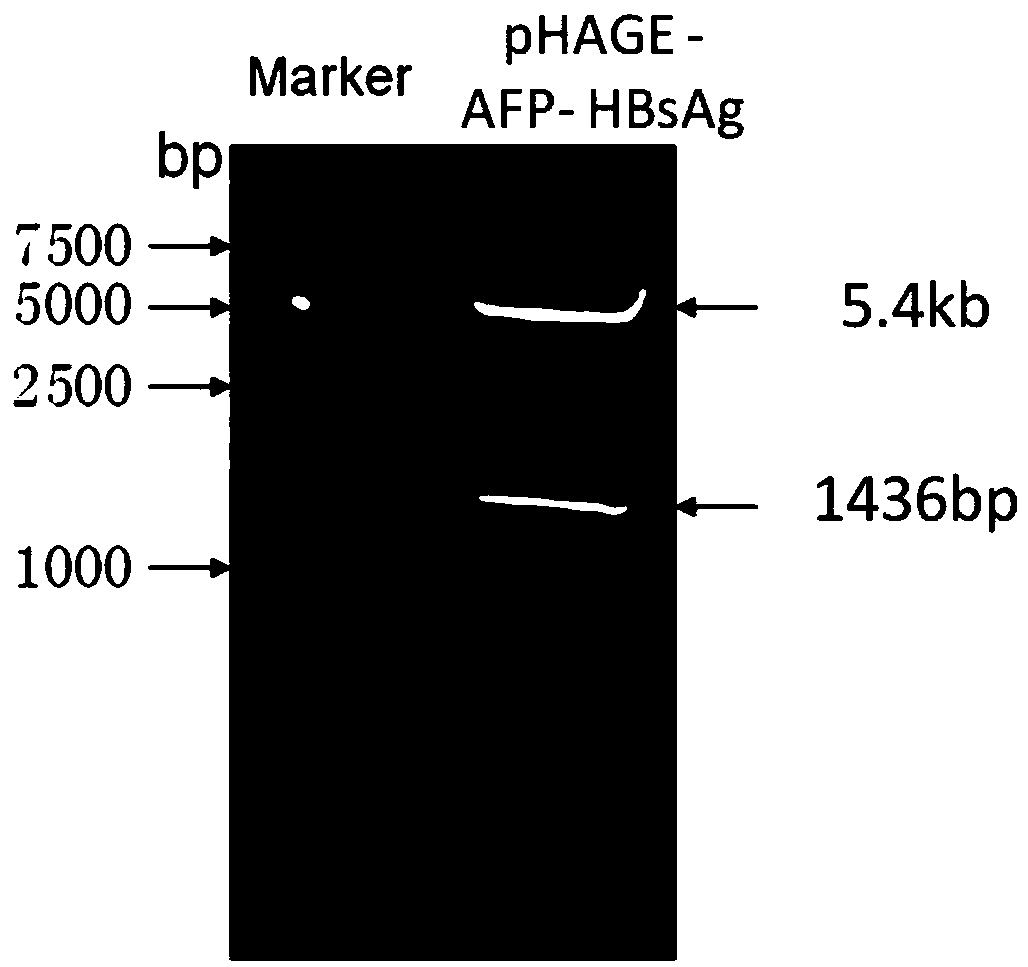
[0055] Using the cancer tissue samples of hepatitis B positive liver cancer patients expressing AFP and HBsAg as a template, PCR amplification was carried out. Among them, P1 and P2 can amplify AFP gene fragments, and P3 and P4 can amplify HBsAg gene fragments. The AFP gene PCR product and the lentiviral expression vector pHAGE-CMV-MCS-IZsGreen were digested with BamH I and Xho I, and the AFP gene fragment and line...
Embodiment 2
[0060] Example 2 Culture of AFP-HBsAg modified DC combined with CIK
[0061] 1. Purification and concentration of pHAGE-AFP-HBsAg lentivirus
[0062] The lentiviral expression plasmid pHAGE-AFP-HBsAg, the packaging plasmid psPAX2 and the envelope plasmid pMD2G were respectively extracted with a plasmid extraction kit, and the concentration of the plasmid was measured by a spectrophotometer. The three plasmids were transfected with calcium phosphate at a mass ratio of 3:2:2. 293T cells were co-transfected with the staining reagent. Collect the virus supernatant in 15ml tubes at 72 hours after transfection, centrifuge at 500G for 10min at 4°C, transfer the supernatant to a new 15ml tube, filter the virus supernatant with a 0.45μm filter; filter the virus supernatant at 4°C, 26000r / min ultracentrifugation for 4 hours, discard the supernatant, and dissolve the pellet in Gibco AIM-V serum-free medium pre-cooled at 4°C overnight to obtain the pHAGE-AFP-HBsAg virus concentrate, whi...
Embodiment 3
[0079] Example 3 In vitro killing of liver cancer cells by DC-CIK
[0080] Take the liver cancer cells HepG2.215 and common liver cancer cells HepG2 stably expressing hepatitis B surface antigen, pre-stain with carboxyfluorescein succinimide (CFSE), and modify the DC-CIK cells with the AFP-HBsAg prepared in Example 2 the next day Co-cultivation was carried out at a ratio of 1:5, 1:10, 1:20, and after 4 hours of co-cultivation, PI was stained. Cell apoptosis was detected by flow cytometry, and the amount of cell death was calculated according to the following formula: death rate=(control-sample) / control×100%, and the control was tumor cells without adding DC-CIK: the sample was adding DC - CIK tumor cells, such as Figure 7 Shown: AFP-HBsAg modified DC-CIK cells have extremely high specific killing activity on hepatitis B-positive liver cancer cells HepG2.215 and common liver cancer cells HepG2, respectively reaching 97.8 ± 10.2% and 78.5±12.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com