Calcium fluoride coated lithium nickel manganate and preparation method thereof
A technology of lithium nickel manganese oxide and calcium fluoride, which is applied in the field of electrochemistry, can solve problems such as insignificant coating effect, electrolyte corrosion, instability, etc., to improve high-temperature electrochemical cycle performance, improve performance, and prevent deintercalation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
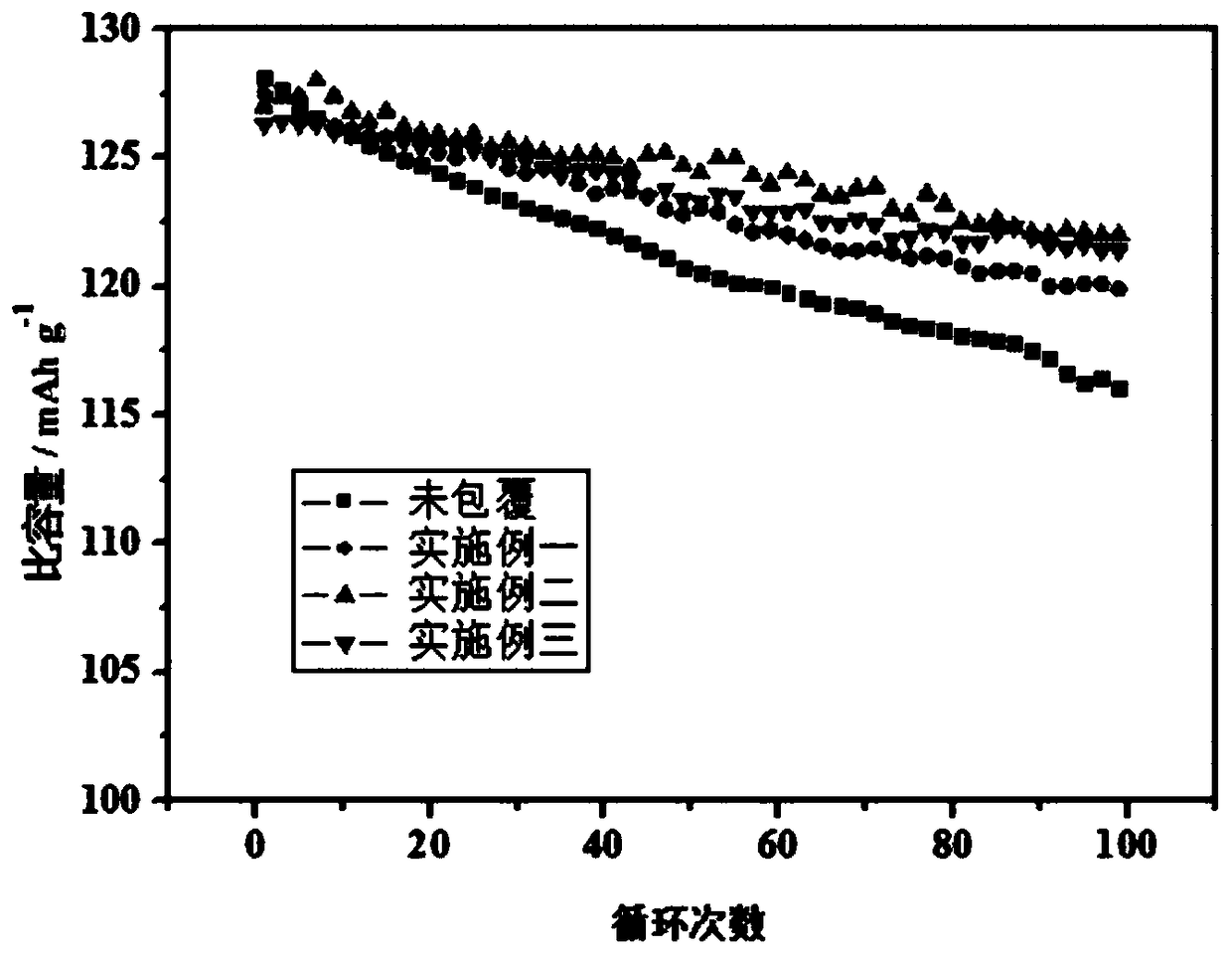
Embodiment 1
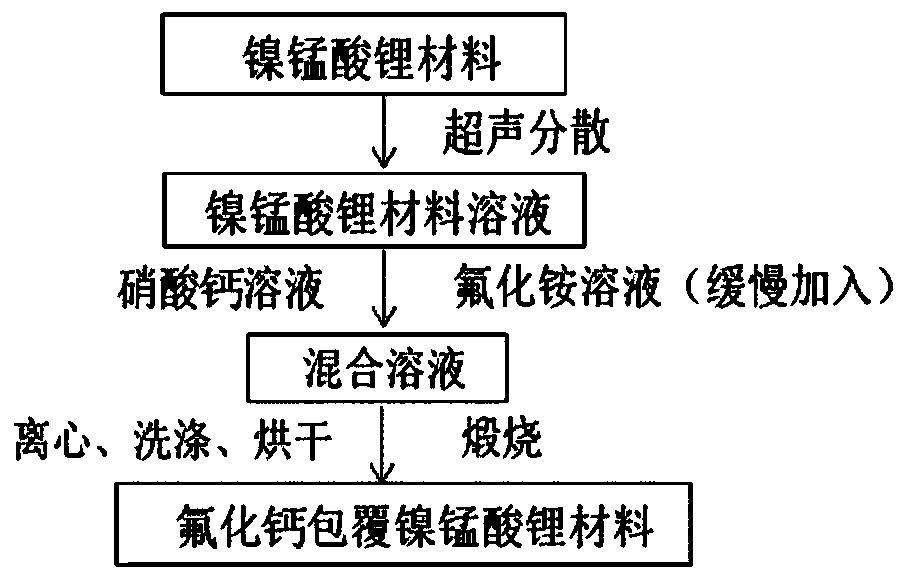
[0041] Such as figure 1 As shown, the preparation process of calcium fluoride-coated lithium nickel manganese oxide material comprises the following steps:
[0042] a) Take 5g of lithium nickel manganese oxide and add it to 250ml of deionized water, and ultrasonically disperse it for 20 minutes to obtain a uniform lithium nickel manganese oxide solution;
[0043] b) Weigh 3.78g of calcium nitrate tetrahydrate and 1.19g of ammonium fluoride according to the chemical molar ratio of 1:2, add them into two 250ml volumetric flasks respectively, and then use deionized water to make up the volume, and wait until the two materials are fully dissolved. A uniform solution of calcium nitrate and ammonium fluoride was obtained.
[0044] c) Take 10ml of calcium nitrate solution and add it to the solution described in step a, and sonicate for 1 hour and stir for 2 hours;
[0045] d) Add 10ml of ammonium fluoride solution dropwise to the solution prepared in step c within 15 minutes, and b...
Embodiment 2
[0048] a) Take 5g of lithium nickel manganese oxide and add it to 250ml of deionized water, and ultrasonically disperse it for 20 minutes to obtain a uniform lithium nickel manganese oxide solution;
[0049] b) Weigh 3.78g of calcium nitrate tetrahydrate and 1.19g of ammonium fluoride according to the chemical molar ratio of 1:2, add them into two 250ml volumetric flasks respectively, and then use deionized water to make up the volume, and wait until the two materials are fully dissolved. A uniform solution of calcium nitrate and ammonium fluoride was obtained.
[0050] c) Take 20ml of calcium nitrate solution and add it to the solution described in step a, and sonicate for 1 hour and stir for 2 hours;
[0051] d) Add 20ml of ammonium fluoride solution dropwise to the solution prepared in step c within 15 minutes, and bathe in water at 80°C for 5 hours;
[0052] e) Centrifuge the above mixed solution and wash it with deionization for 3 times, then dry it at 80°C for 12 hours ...
Embodiment 3
[0054] a) Take 5g of lithium nickel manganese oxide and add it to 250ml of deionized water, and ultrasonically disperse it for 20 minutes to obtain a uniform lithium nickel manganese oxide solution;
[0055] b) Weigh 3.78g of calcium nitrate tetrahydrate and 1.19g of ammonium fluoride according to the chemical molar ratio of 1:2, add them into two 250ml volumetric flasks respectively, and then use deionized water to make up the volume, and wait until the two materials are fully dissolved. A uniform solution of calcium nitrate and ammonium fluoride was obtained.
[0056] c) Take 50ml of calcium nitrate solution and add it to the solution described in step a, and sonicate for 1 hour and stir for 2 hours;
[0057] d) Add 50ml of ammonium fluoride solution dropwise to the solution prepared in step c within 15 minutes, and bathe in water at 80°C for 5 hours;
[0058] e) Centrifuge the above mixed solution and wash it with deionization for 3 times, then dry it at 80°C for 12 hours ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com