Composite preparation containing clopidogrel and aspirin
A technology of clopidogrel tablets and compound preparations, which is applied in the direction of medical preparations containing active ingredients, pill delivery, organic active ingredients, etc., and can solve the problems of reduced disintegration rate and reduced stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
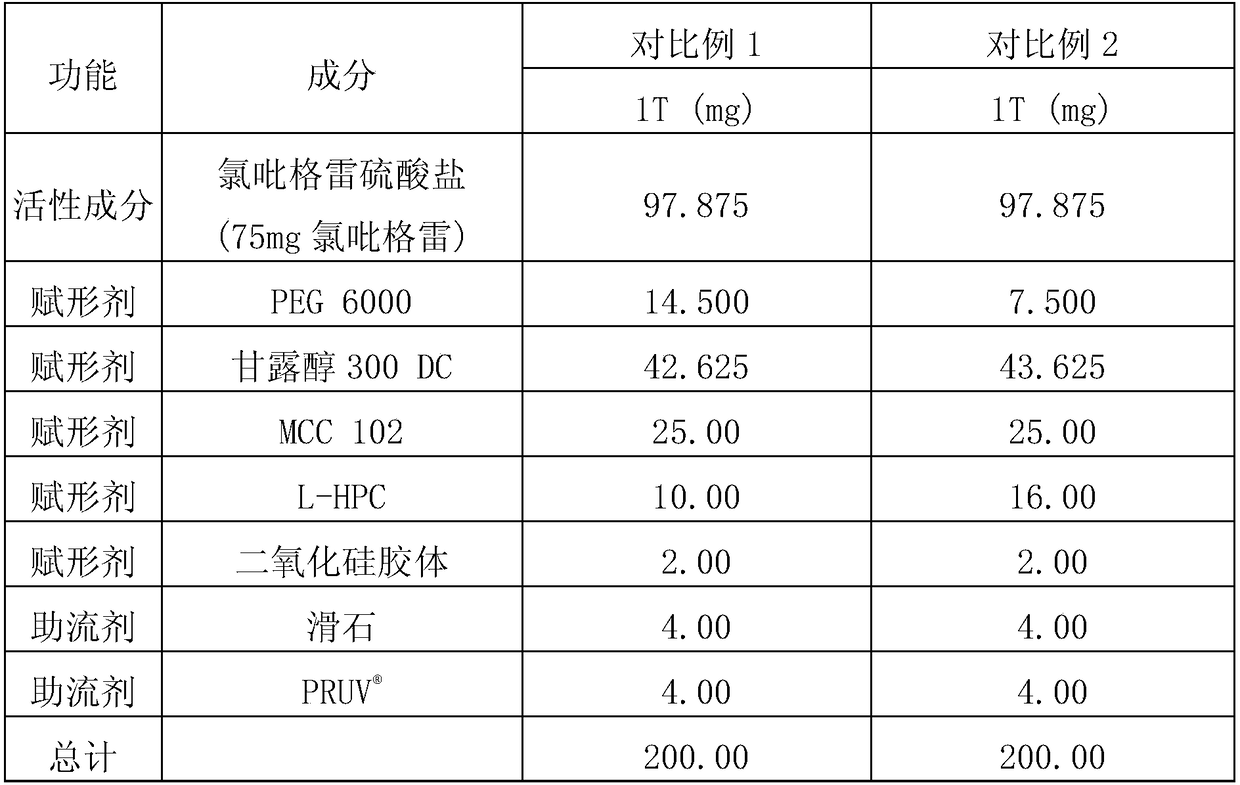
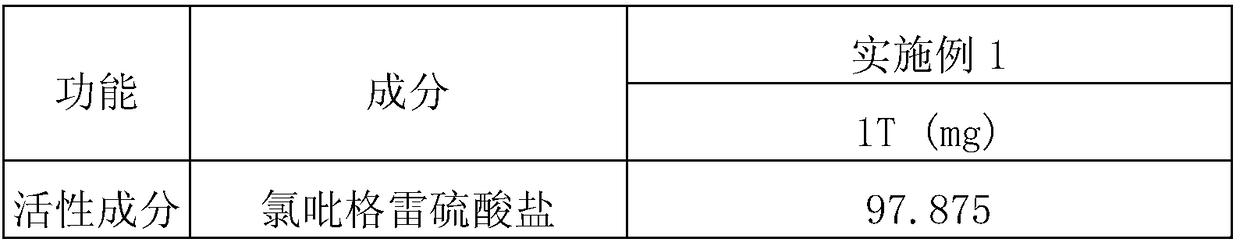
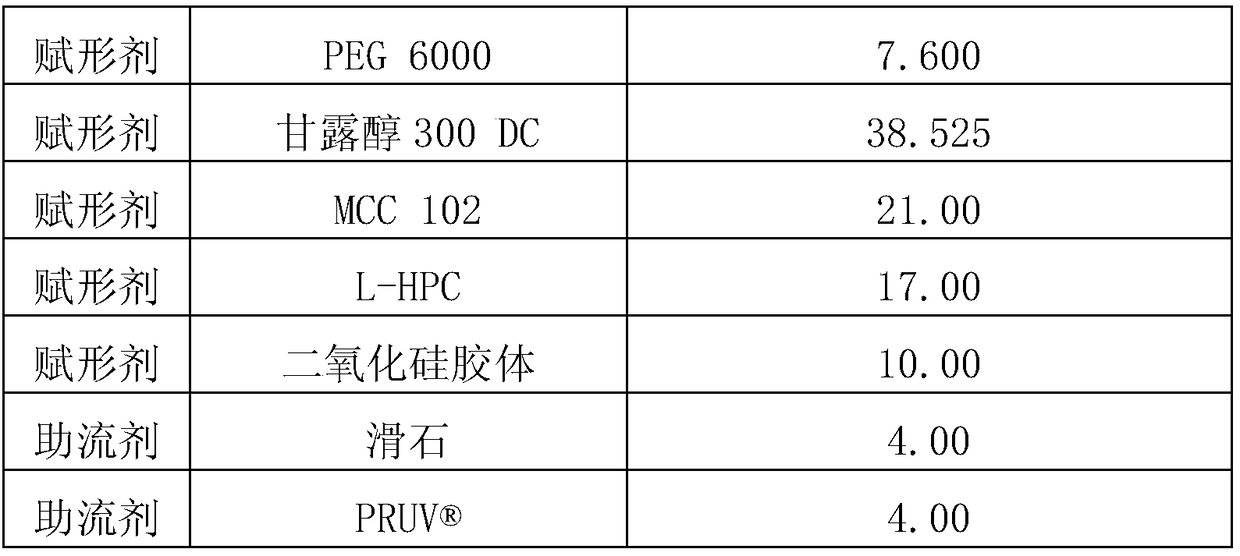
[0086] Tablets were prepared according to the ingredients and contents shown in Table 2 below. Clopidogrel bisulfate and colloidal silica were mixed first, and the mixture was further mixed with PEG 6000, Mannitol 300DC, MCC 102 and L-HPC. Then, add glidants (ie talc and ) and tablet. Each content shown in Table 2 represents the amount per tablet. In particular, the tablets of Example 1 showed no sticking or capping during compression, unlike those prepared in Comparative Examples 1 and 2 (further mixing colloidal silica and using MCC 102). different. The contents of the above ingredients are shown in Table 2 below.
[0087] [Table 2]
[0088]
[0089]
Embodiment 2 to 7
[0091] To further improve sticking and capping phenomena during tableting, MCC112 with low dry weight loss (1.5 wt%) was used. After mixing clopidogrel bisulfate and colloidal silica, the mixture was further mixed with PEG6000, Mannitol 300DC, MCC112, L-HPC, CL-PVP and PVP K-30. Then, add glidant to it And tablet. The content of each ingredient is shown in Table 3 below. Each content shown in Table 3 represents the amount per tablet.
[0092] [table 3]
[0093]
Embodiment 8-10 and comparative example 3-6
[0095] In order to examine the influence of colloidal silica on formulations containing disintegrants, tablets having the compositions shown in Table 4 below were prepared by mainly changing the concentration of colloidal silica in Examples 2-7.
[0096] [Table 4]
[0097]
[0098] Experimental example 1. Disintegration test in Examples 2 to 7
[0099] Disintegration tests were performed on the tablets prepared in Examples 2-7. The disintegration test was performed according to the disintegration test method of the Korean Pharmacopoeia (test solution: water). The results are shown in Table 5 below, and Plavix Used as a control group. It was shown that the disintegration time in Examples 2 to 7 was within 11 minutes, thus showing a decrease compared to the control group. In Examples 2 to 7, tableting could be performed without tableting problems.
[0100] [table 5]
[0101]
[0102] Experimental Example 2. Disintegration tests in Examples 8 to 10 and Comparative Ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com