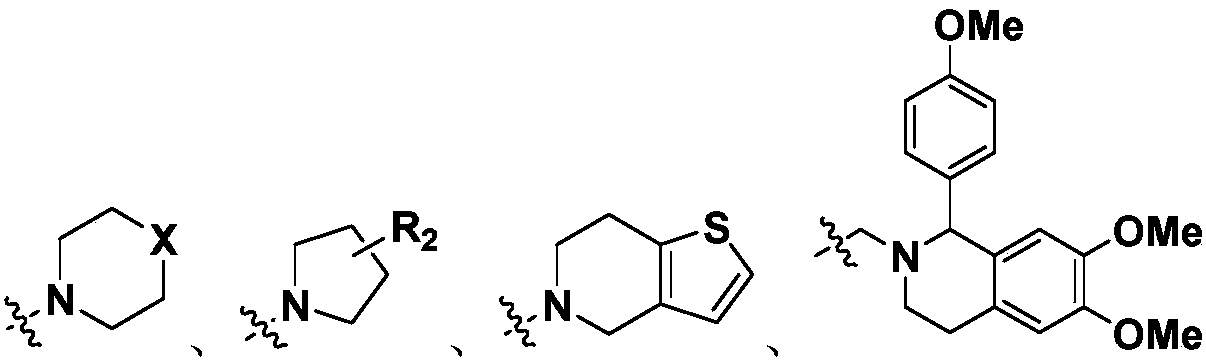
Dendrobium alkaloid derivatives as well as preparation method and medical application thereof
A technology of Dendrobium alkaloids and derivatives, which is applied in the direction of medical formulas, drug combinations, and medical preparations containing active ingredients, etc., and can solve problems such as reports and no biological activity of Pierardine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Synthesis of 3-(2-(dimethylamino)ethyl)-1(3H)-isobenzofuranone (I-1)
[0071]
[0072] Step 1: 2-(3-Oxo-1,3-dihydroisobenzofuran-1-yl)acetic acid (2)
[0073] In a dry 100mL round bottom flask, sequentially add compound 1 (10.0g, 67mmol), oxalic acid (18.0g, 173mmol), sodium acetate (6.0g, 73mmol), add acetic acid (40mL) to dissolve, and raise the temperature under stirring to 100°C and keep warm for 10h. TLC [V (petroleum ether): V (ethyl acetate) = 1:5 as developing solvent] showed that the reaction was almost complete. Add water (60mL) and hydrochloric acid (2.0mol / L) to the reaction solution to adjust the pH value to about 3, and a white solid precipitates, which is filtered, washed with water (20mL×2), and dried in a blast drying oven (60°C) to obtain 6.6 g white solid compound 2, yield 51.6%; mp: 150-152°C.
[0074] Step 2: 3-(2-Hydroxyethyl)isobenzofuran-1(3H)-one (3)
[0075] In a dry 100mL round-bottomed flask, compound 2 (5.35g, 28mmol) and anhydrous te...
Embodiment 2
[0081] With reference to the synthetic method of Example 1, dimethylamine hydrochloride was replaced by tetrahydropyrrole, and a white solid 3-(2-(pyrrolidin-1-yl)isobenzofuran-1(3H)-one was obtained by hydrochloric acid salification Hydrochloride (I-2), yield 73.1%. 1 HNMR (400MHz, D 2 O): δ7.78(dd, J=7.9, J=1.1, 1H), 7.70(td, J=7.7, J=1.1, 1H), 7.50~7.55(m, 2H), 5.67(dd, J= 8.5, J= 3.2, 1H), 3.47~3.64(s, 2H), 3.12~3.32(m, 2H), 2.86~3.02(m, 2H), 2.94(s, 2H), 2.47~2.59(m, 1H ),2.05~2.17(m,1H),1.99(s,2H),1.88(s,2H); 13 CNMR (100MHz,D 2 O)δ: 173.06, 148.54, 135.33, 129.79, 125.40, 124.43, 122.13, 79.87, 54.37, 54.08, 50.91, 29.85, 22.54; ESI-MS: m / z=232.2[M+H] + .
Embodiment 3
[0083] With reference to the synthetic method of Example 1, dimethylamine hydrochloride was replaced by tetrahydrothienopyridine, and a white solid 3-(2-(6,7-dihydrothieno[3,2-c]pyridine was obtained through hydrochloric acid salification) -5(4H)-yl)ethyl)isobenzofuran-1(3H)-one hydrochloride (I-3), yield 63.5%. 1 H-NMR (400MHz, DMSO-d 6 ): δ11.36(s,1H),7.81~7.93(m,2H),7.76(d,J=7.8,1H),7.66(t,J=6.9,1H),7.49(d,J=5.3, 1H),6.86~6.95(m,1H),5.75~5.86(m,1H),4.47~4.59(m,1H),4.12~4.29(m,1H),3.74~3.87(m,1H),3.17~ 3.54(m,2H),3.39(s,2H),3.01~3.17(m,1H),2.72~2.86(m,1H),2.16~2.34(m,1H); 13 C-NMR (100MHz, DMSO-d 6 ))δ: 169.94, 149.51, 135.12, 131.83, 130.13, 128.60, 125.67, 125.62, 125.58, 125.47, 123.22, 79.03, 51.86, 51.80, 50.70, 50.62, 49.53, 499.42,2 z=300.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com