Preparation method of cisatracurium besilate injection without preservatives
A technology of cisatracurium besylate and injection, which is applied in the field of medicine and can solve the problems of easy generation of impurities and increased content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0018] Find after research: (1) the main degradation impurity of cisatracurium besylate is cis-acid, R-laudane base, cis-hydroxyl compound and cis-acrylic acid mixture, and cis-atracurium besylate is not stable under high temperature conditions. Stable, 60 ℃ treatment 8h, cis-acid, R-laudane base, cis-hydroxy compound and cis-acrylic acid mixture increased by 1.11%, 5.15%, 1.40% and 4.65%, respectively.
[0019] (2) cisatracurium besylate is unstable under acidic conditions. After being treated with 0.1mol / L hydrochloric acid, the content of R-laudansine rises to 16.2%, but it is extremely unstable under alkaline conditions and can be neutralized immediately , R-laudanline increased by 8.87%, and the content of cis-acrylic acid mixture was 6.03%. Choosing an appropriate pH is beneficial to ensure the stability of cisatracurium besylate injection.
[0020] (3) cisatracurium besylate is relatively stable under oxidation conditions, 30% H 2 o 2 conditions, the cis-acid and cis-...
specific Embodiment 1
[0026] Element
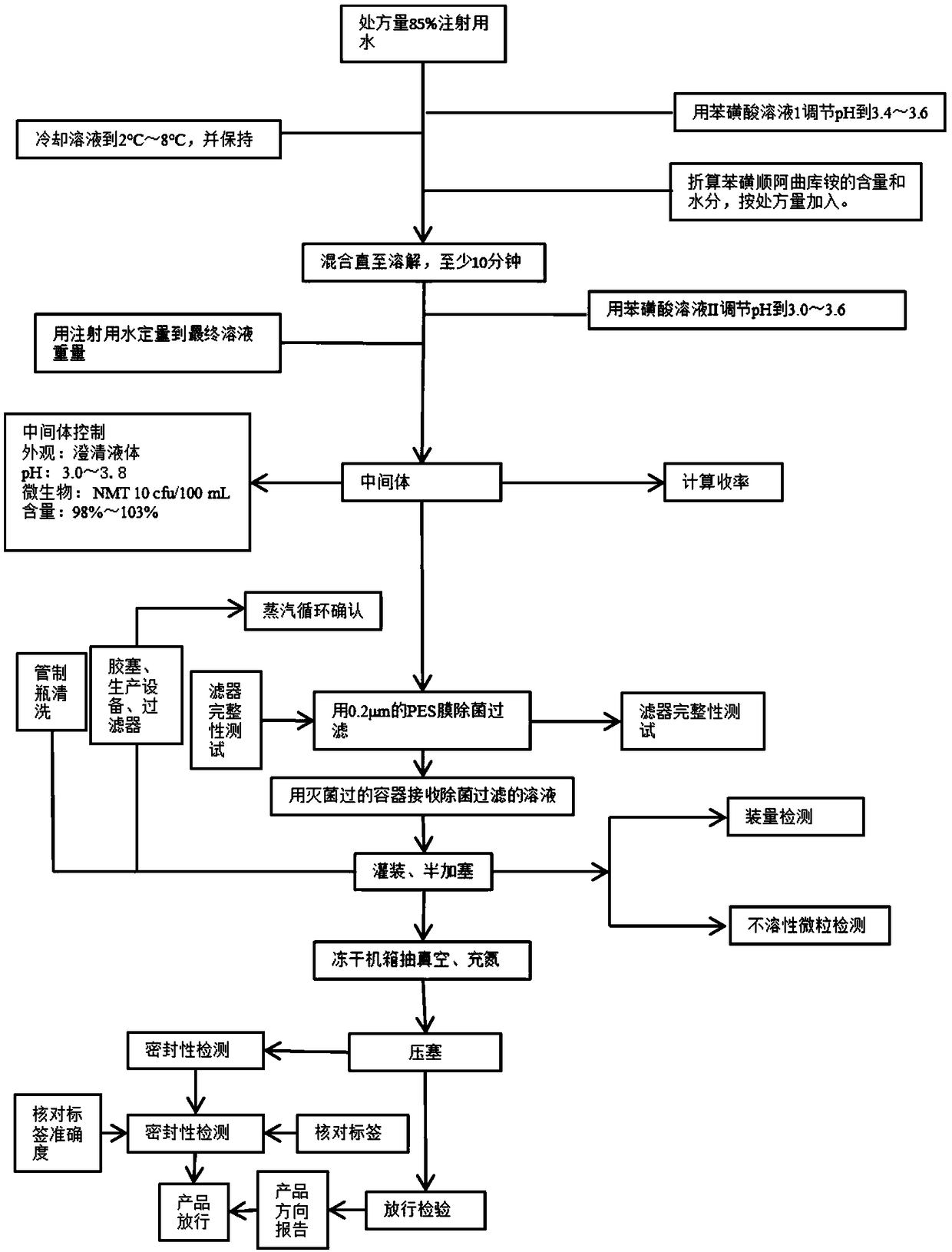
[0027] The preparation method is: measure about 850g of water for injection, adjust the pH to 3.4-3.6 with benzenesulfonic acid solution I, keep warm at 2°C-8°C to obtain solution A, add the prescribed amount of cisatracurium besylate into solution A , stir and dissolve to obtain solution B, adjust the pH of solution B to 3.0-3.6 with benzenesulfonic acid solution II, add water for injection to 1000.0g, and obtain solution C; pass solution C through a 0.22 μm polyethersulfone membrane, and pour Pack 5ml into a freeze-drying box, vacuumize at 2°C to 8°C, fill with nitrogen, press the stopper, and crimp the cap.
specific Embodiment 2
[0028] Element
[0029] The preparation method is: measure about 850g of water for injection, adjust the pH to 3.4-3.6 with benzenesulfonic acid solution I, keep warm at 2°C-8°C to obtain solution A, add the prescribed amount of cisatracurium besylate into solution A , stir and dissolve to obtain solution B, adjust the pH of solution B to 3.0-3.6 with benzenesulfonic acid solution II, add water for injection to 1000.0g, and obtain solution C; pass solution C through a 0.22 μm polyethersulfone membrane, and pour Pack 5ml into a freeze-drying box, vacuumize at 2°C to 8°C, fill with nitrogen, press the stopper, and crimp the cap.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com