AIE (aggregation-induced emission) compound as well as preparation method and application thereof
A compound and chemical formula technology, applied in the field of organic compounds and their preparation, can solve the problems of cumbersome preparation process and difficult separation, and achieve the effects of simple preparation process, operation of reaction conditions and fast growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
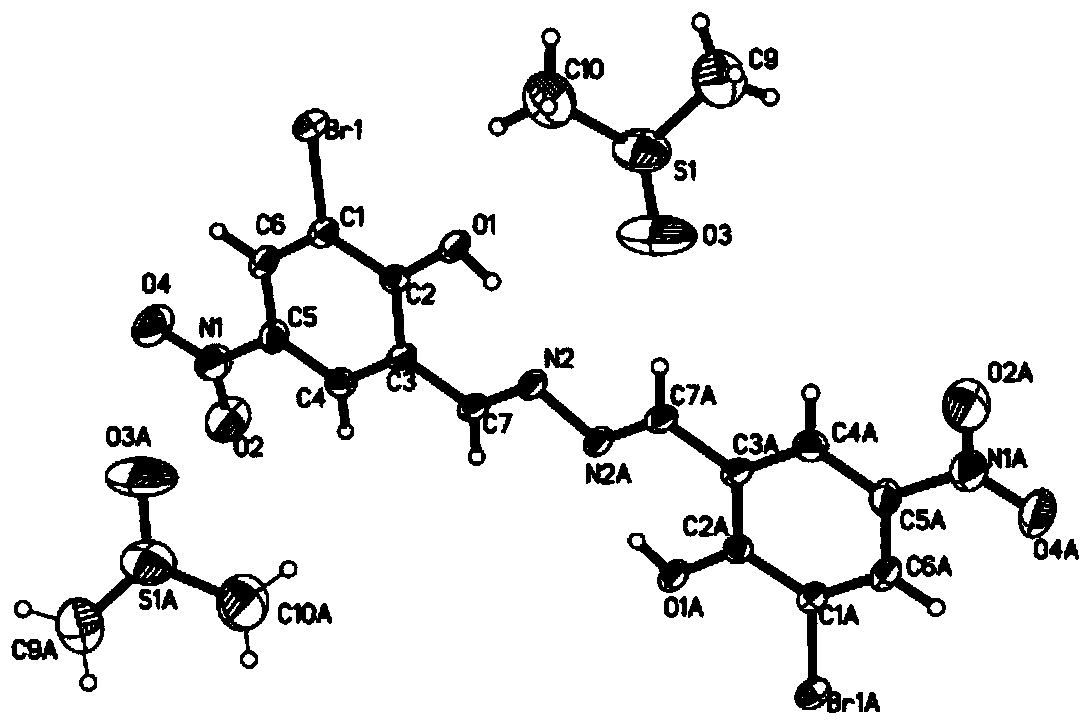
[0022] Embodiment 1. Measure 40 milliliters of absolute ethanol, dissolve 2.4868 grams (10 mmol) of 3-bromo-5-nitrosalicylaldehyde, and in a round-bottomed flask, the solution is reddish-yellow; measure 61 μL (2 mmol) of hydrazine hydrate, Add dropwise to the above solution, stir, add a drop of 6mol / L sulfuric acid, heat and reflux for 2 hours, stop heating, wait for the reaction temperature to drop to room temperature, vacuum filter the reactant, water / ethanol mixed solvent (volume ratio 1:2) The precipitate was washed and filtered with suction to obtain a pale yellow solid, which was dried. The light yellow solid was dissolved in dimethyl sulfoxide (DMSO) solution, sealed and left standing, after several hours, orange needle-like crystals were precipitated. 1 HNMR (600MHz, DMSO-d 6 ) (ppm) 9.262 (s, 2H, -HC=N-), 8.658 (s, 2H, Ph-H), 8.551 (s, 2H, Ph-H). Electrospray mass spectrometry results (m / z), found: 486.87 (100%), calcd: [M-H] - 486.87. Collected by filtration in 6...
Embodiment 2
[0032] Example 2. Measure 40 milliliters of absolute ethanol, dissolve 2.9842 grams (12 mmol) of 3-bromo-5-nitrosalicylaldehyde, and in a round-bottomed flask, the solution is reddish-yellow; measure 61 μL (2 mmol) of hydrazine hydrate, Add dropwise to the above solution, stir, add a drop of 6mol / L sulfuric acid, heat and reflux for 2 hours, stop heating, wait for the reaction temperature to drop to room temperature, vacuum filter the reactant, water / ethanol mixed solvent (volume ratio 1:2) The precipitate was washed and filtered with suction to obtain a pale yellow solid, which was dried. The light yellow solid was dissolved in dimethyl sulfoxide (DMSO) solution, sealed and left standing, after several hours, orange needle-like crystals were precipitated. 1 HNMR (600MHz, DMSO-d 6 ) (ppm) 9.262 (s, 2H, -HC=N-), 8.658 (s, 2H, Ph-H), 8.551 (s, 2H, Ph-H). Electrospray mass spectrometry results (m / z), found: 486.87 (100%), calcd: [M-H] - 486.87. Collected by filtration, yield ...
Embodiment 3
[0033] Embodiment 3. Measure 40 milliliters of absolute ethanol, dissolve 2.9842 grams (12 mmol) of 3-bromo-5-nitrosalicylaldehyde, and in a round bottom flask, the solution is reddish-yellow; Measure hydrazine hydrate 76 μ L (2.5 mmol) , added dropwise in the above solution, stirred, added a drop of 6mol / L sulfuric acid, heated to reflux for 2 hours, then stopped heating, until the reaction temperature dropped to room temperature, vacuum filtered the reactant, water / ethanol mixed solvent (volume ratio 1:2 ) to wash the precipitate, filter with suction to obtain a pale yellow solid, and dry. The light yellow solid was dissolved in dimethyl sulfoxide (DMSO) solution, sealed and left standing, after several hours, orange needle-like crystals were precipitated. 1 H NMR (600MHz, DMSO-d 6 ) (ppm) 9.262 (s, 2H, -HC=N-), 8.658 (s, 2H, Ph-H), 8.551 (s, 2H, Ph-H). Electrospray mass spectrometry results (m / z), found: 486.87 (100%), calcd: [M-H] - 486.87. Collected by filtration in 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com