Pyrazine-based D-A-D type fluorescent small molecule, preparation method and applications thereof
A D-A-D, small molecule technology, applied in the field of fluorescent probes, can solve the problems of poor dye stability, easy to detach from cells, poor photostability, etc., and achieve the effects of excellent photostability, not easy to quench, and high fluorescence quantum yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
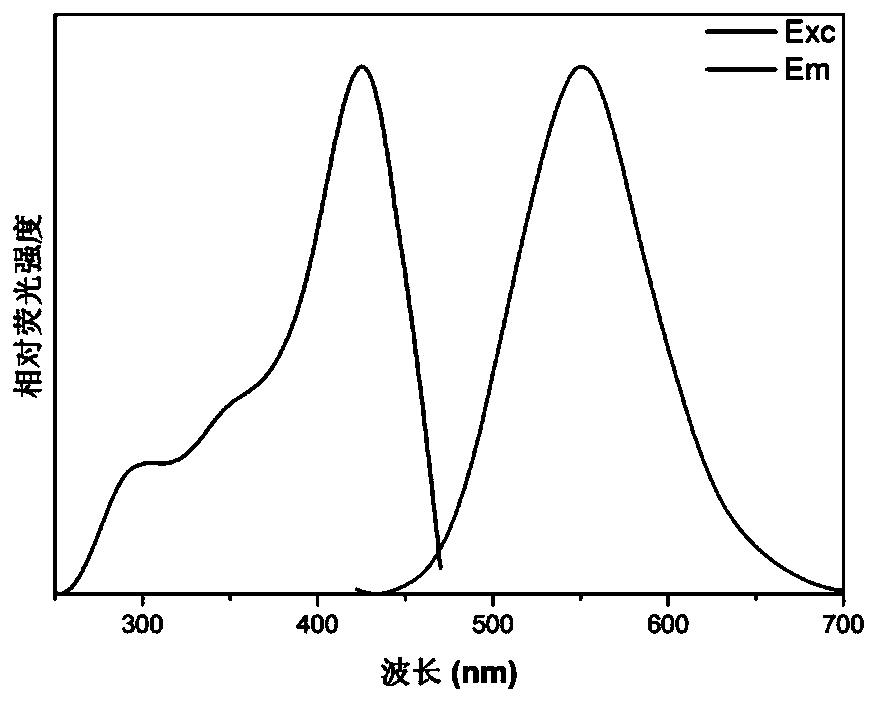
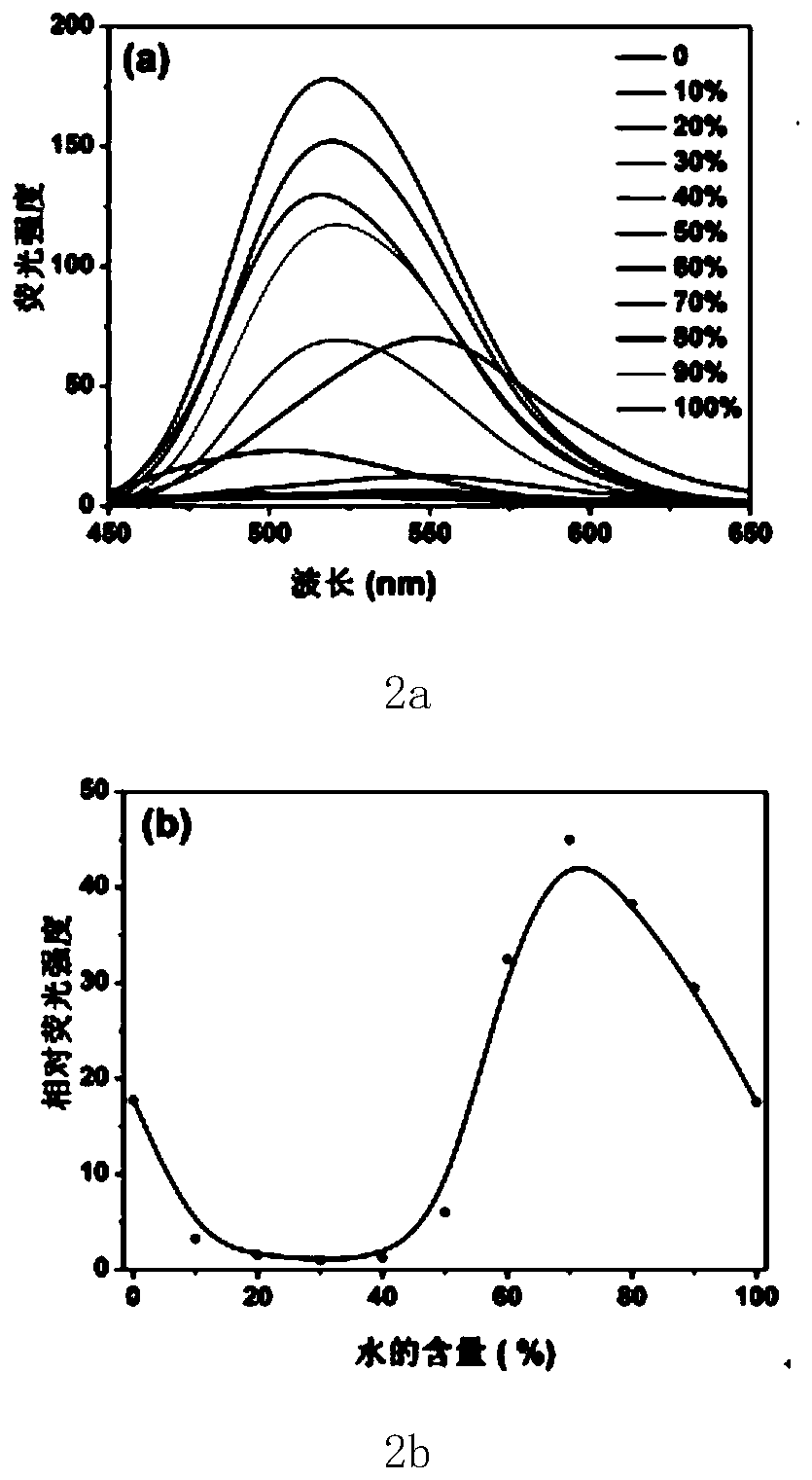
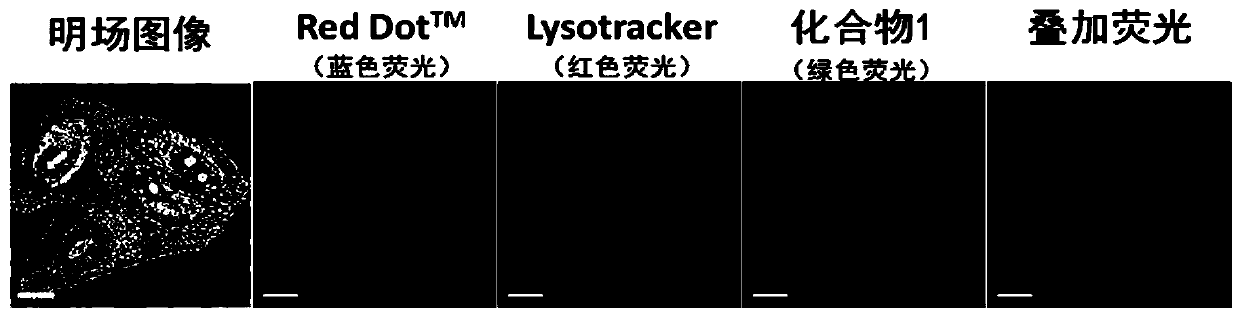
[0033] The fluorescent small molecule 4,4'-(pyrazine-2,5-diyl)bis(N,N-bis(4-methoxyphenyl)aniline) is labeled as compound 1, and its preparation method and detection process are as follows:
[0034] (1) Weigh 242 mg of 3,6-dibromopyrazine, 4-methoxy-N-(4-methoxyphenyl)-N-(4-(4,4,5,5-tetramethyl -1,3,2-dioxaborolan-2-yl)phenyl)aniline 1.29g, Pd 2 (dba) 3 127mg, sphos88mg in 50ml reaction tube, in N 2 Add Cs under protection 2 CO 3 Solution (4mol / L) 5ml and toluene 10ml, react at 85°C for 24h. The reaction product was washed with water, extracted three times with dichloromethane, and the organic phase was collected. Purify by silica gel column chromatography to obtain 402 mg of yellow solid 4,4'-(pyrazine-2,5-diyl)bis(N,N-bis(4-methoxyphenyl)aniline) with a yield of 58%. PD 2 (dba) 3 , sphos and Cs 2 CO 3 All solutions are catalysts. The structural formula of compound 1 is as follows:
[0035]
[0036] Its NMR spectrum data: 1 HNMR (400MHz, CDCl3): δ=3.81(s, 12H)...
Embodiment 2
[0046] (1) The preparation process of the fluorescent small molecule 4,4'-(3-fluoropyrazine-2,5-diyl)bis(N,N-diphenylaniline) is as follows: the fluorescent small molecule is named compound 2;
[0047] First prepare 2,5-dibromo-3-fluoropyrazine, specifically, weigh 2.53 g of 2-amino-3,6-dibromopyrazine and NaNO 2 1.38g in a 100ml round bottom flask, slowly add HBF dropwise at 0°C 4 25ml, reacted at 20°C for 2h. The resulting reaction mixture was washed with water and extracted three times with dichloromethane, and the organic phase was collected. The resulting organic phase was purified by silica gel column chromatography to obtain 2,5-dibromo-3-fluoropyrazine. Then, 140 mg of the obtained 2,5-dibromo-3-fluoropyrazine, 523 mg of 4-(diphenylamino) phenylboronic acid pinacol ester, and 119 mg of bis(triphenylphosphine)palladium(II) dichloride were taken, Tetrabutylammonium bromide 142mg in 50ml reaction tube, in N 2 Join K under protection 2 CO 3 Solution (6mol / L) 5ml and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com