A kind of AIE compound and its preparation method and application
A compound and chemical formula technology, applied in the field of organic compounds and their preparation, can solve problems such as difficult separation and cumbersome preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
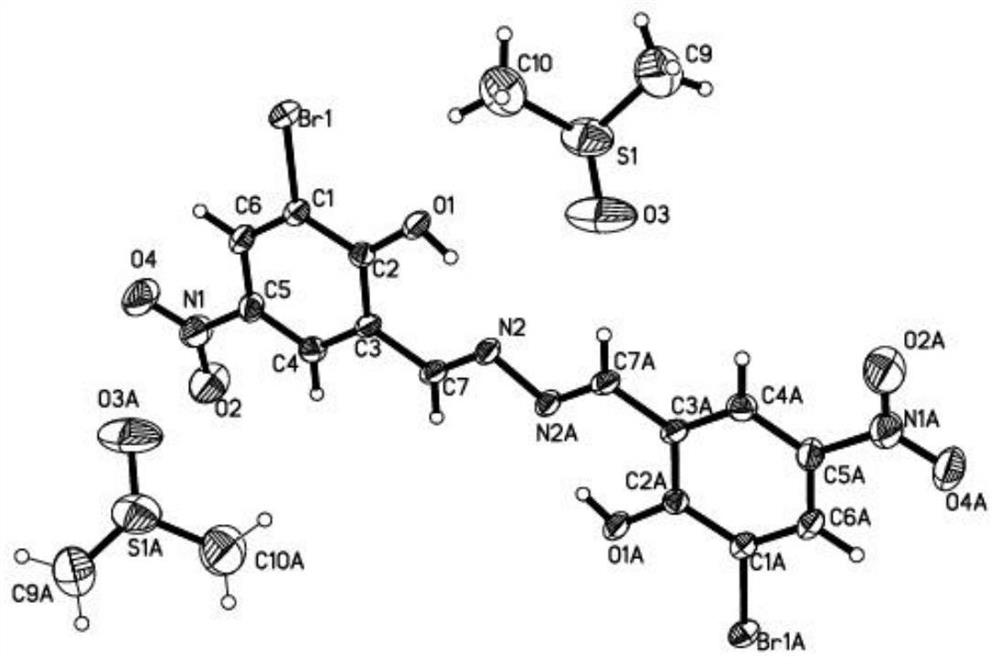
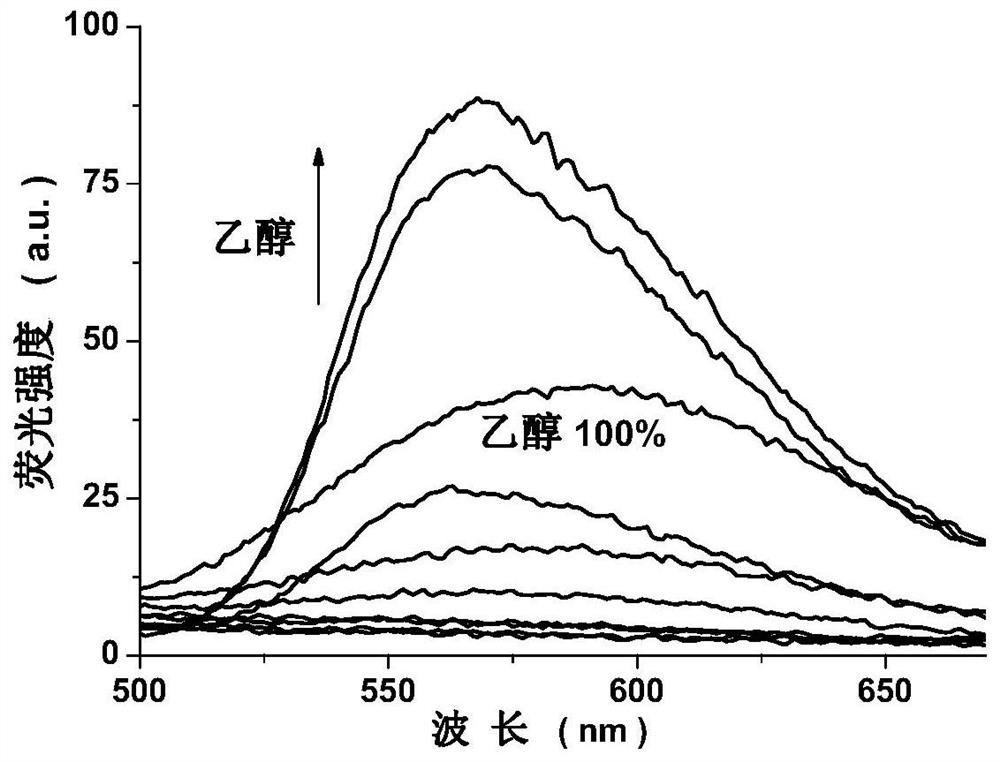
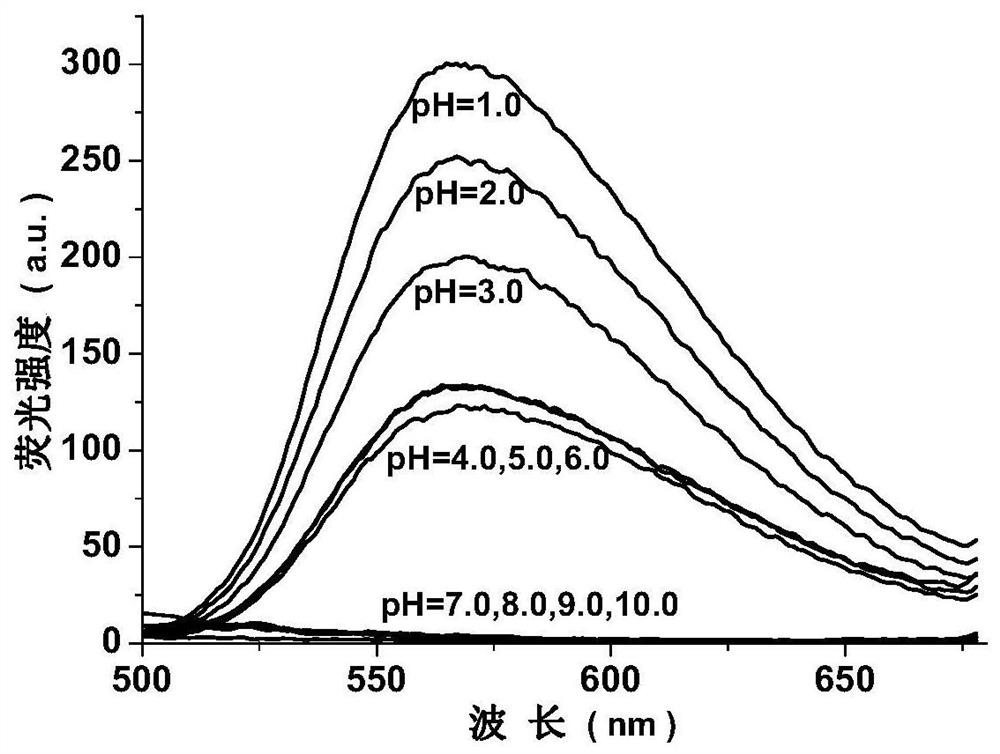
[0022]Example 1. EtOAc EtOAc (EtOAc) EtOAc (EtOAc) EtOAc (EtOAc) EtOAc (EtOAc) EtOAc (EtOAc) EtOAc (EtOAc) The solution is red and yellow in the round bottom flask, and the solution is red-yellow; 2 mmol, Add it to the above solution, stirred, stirred with 6 mol / L sulfuric acid, heated and refluxed for 2 hours, stop heating, and the temperature is lowered to room temperature, vacuum filtration reactants, water / ethanol mixed solvent (volume ratio 1: 2) The precipitate was washed, filtered to give a light yellow solid, dried. The pale yellow solid was dissolved in the dimethyl sulfoxide (DMSO) solution, and the seal was sealed, and the orange needle crystal was precipitated after several hours.1HNMR (600MHz, DMSO-D6(PPM) 9.262 (S, 2H, -HC = N -), 8.658 (S, 2H, pH-H), 8.551 (S, 2H, pH-H). Electronic spray mass spectrometry (m / z), Found: 486.87 (100%), CALCD: [M-H]-486.87. Filter collection, yield is 68%.
[0023]The structure of the compound:
Embodiment 2
[0032]Example 2. Quantity was removed from 40 ml of anhydrous ethanol, dissolved 3-bromo-5-nitrogen hydrarsedehyde 2.9842 g (12 mmol), in the round bottom flask, and the solution was red and yellow; 61 μl of hydrazine (2 mmol), Add it to the above solution, stirred, stirred with 6 mol / L sulfuric acid, heated and refluxed for 2 hours, stop heating, and the temperature is lowered to room temperature, vacuum filtration reactants, water / ethanol mixed solvent (volume ratio 1: 2) The precipitate was washed, filtered to give a light yellow solid, dried. The pale yellow solid was dissolved in the dimethyl sulfoxide (DMSO) solution, and the seal was sealed, and the orange needle crystal was precipitated after several hours.1HNMR (600MHz, DMSO-D6(PPM) 9.262 (S, 2H, -HC = N -), 8.658 (S, 2H, pH-H), 8.551 (S, 2H, pH-H). Electronic spray mass spectrometry (m / z), Found: 486.87 (100%), CALCD: [M-H]-486.87. Filter collection, yield is 50%.
Embodiment 3
[0033]Example 3. Substated 40 ml of anhydrous ethanol, dissolved 3-bromo-5-nitrogen hydrarse aldehyde 2.9842 g (12 mmol), in the round bottom flask, the solution was red and yellow; the amount of hydrazine was 76 μL (2.5 mmol) The above solution was added dropwise, stirred, stirred, add 6 mol / L sulfuric acid, and the heating was refluxed after 2 hours, stop heating, and the temperature was lowered to room temperature, vacuum filtration reactants, mixed with water / ethanol (volume ratio 1: 2) ) Wash the precipitate, remove filtration, to obtain a light yellow solid, dried. The pale yellow solid was dissolved in the dimethyl sulfoxide (DMSO) solution, and the seal was sealed, and the orange needle crystal was precipitated after several hours.1H NMR (600MHz, DMSO-D6(PPM) 9.262 (S, 2H, -HC = N -), 8.658 (S, 2H, pH-H), 8.551 (S, 2H, pH-H). Electronic spray mass spectrometry (m / z), Found: 486.87 (100%), CALCD: [M-H]-486.87. Filter collection, yield is 30%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com