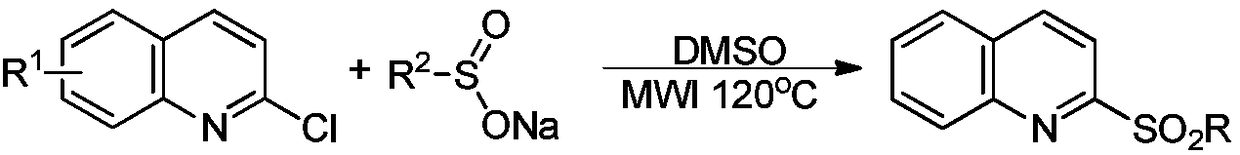
Synthetic method for 2-aliphatic sulfonyl quinoline derivative
The technology of a family of sulfonylquinolines and synthesis methods is applied in the field of synthesis of 2-aliphatic sulfonylquinolines, which can solve the problems of harsh reaction conditions, increased operation steps, and high cost of raw materials in the synthesis method, so as to avoid environmental problems and reduce impact , the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 2-Benzylsulfonylquinoline:
[0036]
[0037] In a 50mL round-bottomed flask, add 1.64g of 2-chloroquinoline, 2.10g of benzylsulfonyl chloride, 2.09g of potassium sulfite, and 20ml of water in sequence, and react ultrasonically for 10 minutes in a 60W / 160KHz ultrasonic reaction device. The crude product of 2-benzylsulfonylquinoline was obtained by filtration, and the crude product was washed with 95% ethanol to obtain 2.60 g of the corresponding pure product, with a yield of 92%. NMR data:
[0038] 1 H NMR (400MHz, CDCl 3 ):δ=8.32–8.28(m,2H),7.92–7.86(m,3H),7.73(t,J=8.0Hz,1H),7.24–7.19(m,5H),4.80(s,2H); 13 C NMR (100MHz, CDCl 3 ): δ=156.2, 147.1, 138.4, 131.2, 131.1, 130.1, 129.3, 129.1, 128.6, 128.6, 127.9, 127.4, 117.9, 58.2.
[0039] Control group 1:
[0040] Replace ultrasonic-assisted reactions with room temperature stirred reactions:
[0041] In a 50 mL round bottom flask, 1.64 g of 2-chloroquinoline, 2.10 g of benzylsulfonyl chloride, 2.09 g of potassium...
Embodiment 2
[0067] 2-bromoquinoline is the synthesis of 2-benzylsulfonylquinoline as raw material:
[0068] In a 50mL round-bottom flask, add 2.08g of 2-bromoquinoline, 2.10g of benzylsulfonyl chloride, 2.09g of potassium sulfite, 20ml of water, and react ultrasonically for 10 minutes in a 60W / 160KHz ultrasonic reaction device. The crude product of 2-benzylsulfonylquinoline was obtained by filtration, and the crude product was washed with 95% ethanol to obtain 2.57 g of the corresponding pure product, with a yield of 92%.
Embodiment 3
[0070] 2-iodoquinoline is the synthesis of 2-benzylsulfonylquinoline as raw material:
[0071] In a 50mL round-bottomed flask, add 2.55g of 2-iodoquinoline, 2.10g of benzylsulfonyl chloride, 2.09g of potassium sulfite, 20ml of water, and react ultrasonically for 10 minutes in a 60W / 160KHz ultrasonic reaction device. 2-benzylsulfonylquinoline was obtained by filtration: the crude product was washed with 95% ethanol to obtain 2.60 g of the corresponding pure product with a yield of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com