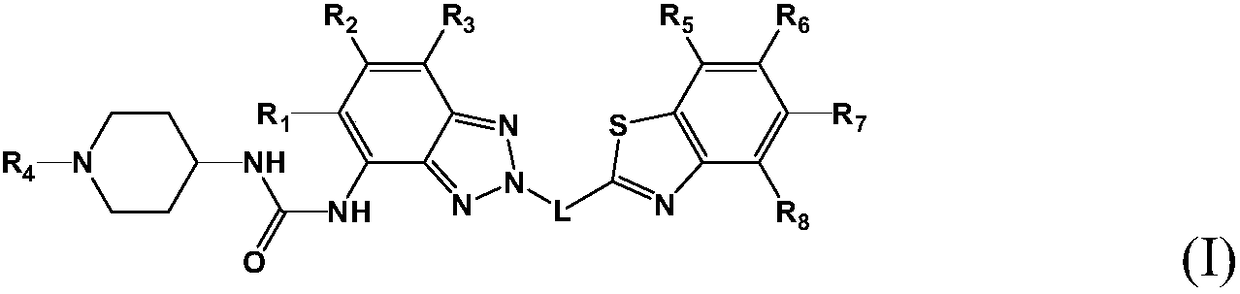
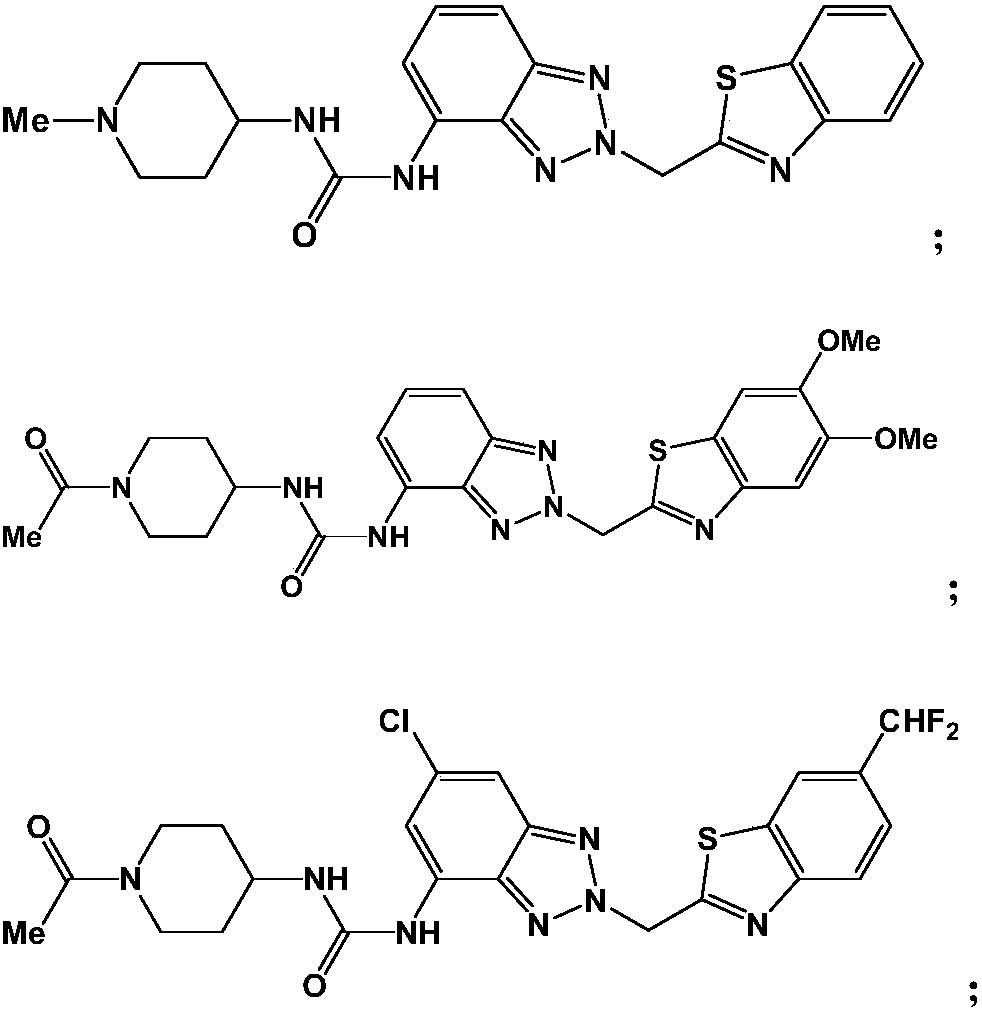
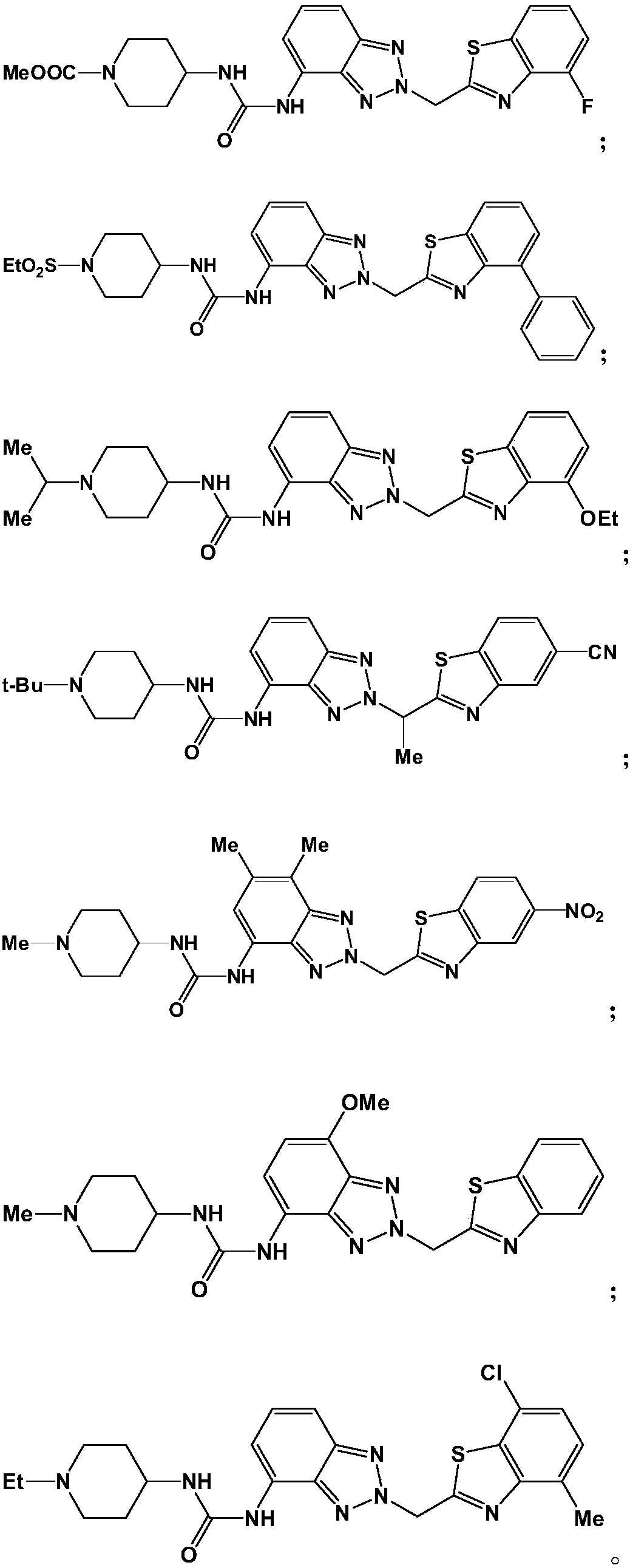
Antitumor drug and preparation method thereof
A pharmaceutical and prodrug technology, applied in the field of medicine, can solve problems such as unsatisfactory curative effect, difficulty in obtaining therapeutic effect, and damage to patient immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: the synthesis of compound 1
[0055]
[0056] Step 1: 2H-benzo[d][1,2,3]triazol-4-amine (10mmol) and 2-(bromomethyl)benzo[d]thiazole (10mmol), potassium carbonate (20mmol) The aqueous solution and 100ml of ethanol were added into a round-bottomed flask, stirred and heated to reflux under nitrogen protection, and reacted overnight. After the reaction solution was cooled and filtered, 200ml of ethyl acetate was added, then washed with water, and the organic layer was dried over anhydrous sodium sulfate. After the solvent was evaporated under reduced pressure, it was separated by silica gel column chromatography (petroleum ether:ethyl acetate=20:1) to obtain Intermediate 2-(benzo[d]thiazol-2-ylmethyl)-2H-benzo[d][1,2,3]triazol-4-amine 2.08g, yield 74.2%. ESI-MS: 282.34[M+H] +
[0057] Step 2: Add 2-(benzo[d]thiazol-2-ylmethyl)-2H-benzo[d][1,2,3]triazol-4-amine (5mmol), 4-isocyanato- 1-Methylpiperidine (5 mmol) was dissolved in 100 ml of dichloromethane...
Embodiment 2
[0062] Embodiment 2: the synthesis of compound 2
[0063]
[0064] Step 1: Mix 2H-benzo[d][1,2,3]triazol-4-amine (10mmol) and 2-(bromomethyl)-5,6-dimethoxybenzo[d]thiazole (10mmol), an aqueous solution of potassium carbonate (20mmol), and 100ml of ethanol were added into a round-bottomed flask, stirred and heated to reflux under nitrogen protection, and reacted overnight. After the reaction solution was cooled and filtered, 200ml of ethyl acetate was added, then washed with water, and the organic layer was dried over anhydrous sodium sulfate. After the solvent was evaporated under reduced pressure, it was separated by silica gel column chromatography (petroleum ether:ethyl acetate=30:1) to obtain Intermediate 2-(5,6-dimethoxy-benzo[d]thiazol-2-ylmethyl)-2H-benzo[d][1,2,3]triazol-4-amine 2.34g , yield 69.3%. ESI-MS: 341.39[M+H] +
[0065] Step 2: 2-(5,6-dimethoxy-benzo[d]thiazol-2-ylmethyl)-2H-benzo[d][1,2,3]triazol-4-amine (5 mmol), 1-(4-isocyanatopiperidin-1-yl)ethano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com