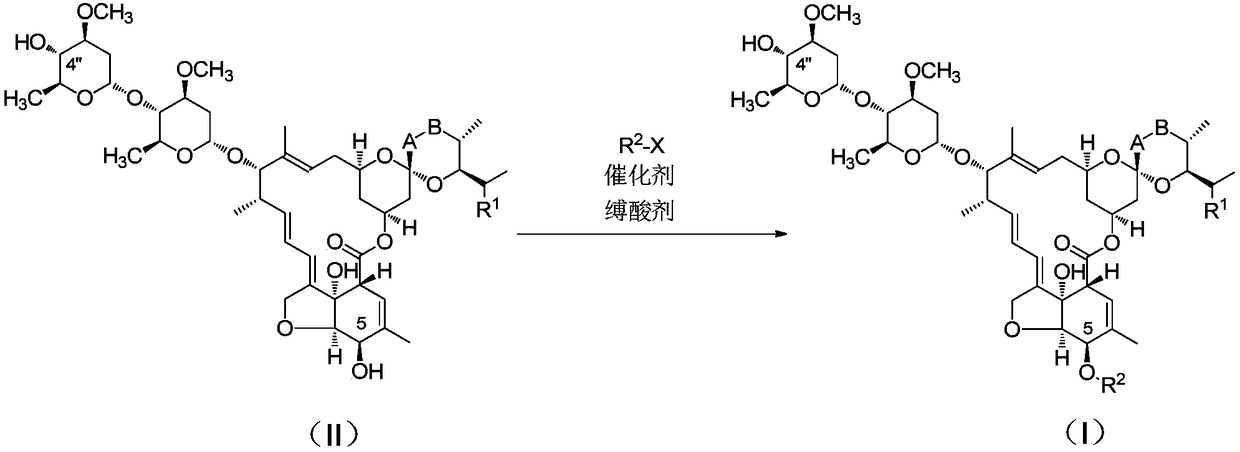
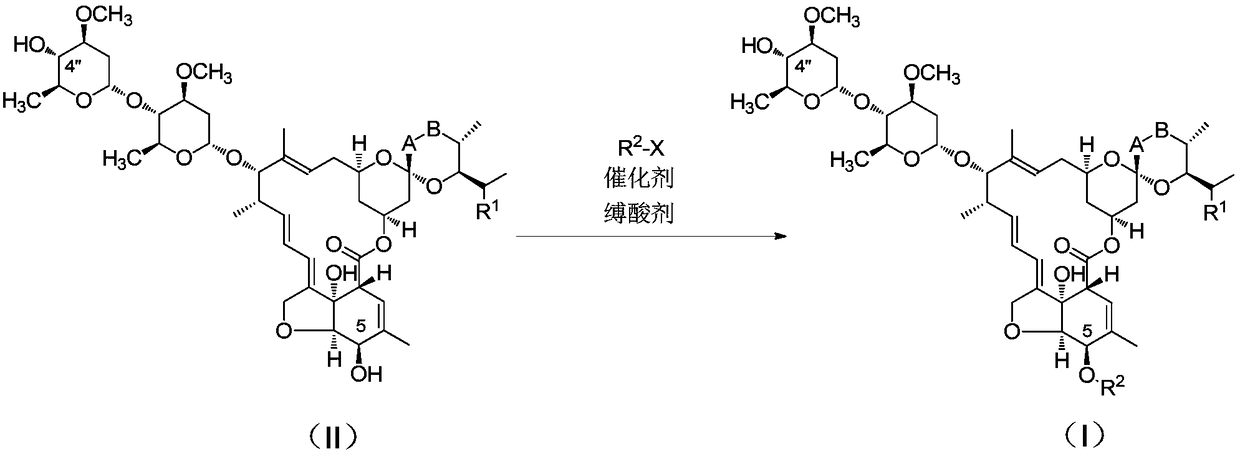
Catalytic synthesis process of key intermediate of emamectin
A technology of methylamino abamectin and synthesis process, applied in the directions of organic chemistry, organic chemical methods, sugar derivatives, etc., can solve the problems of low yield, high pollution, poor selectivity, etc., achieve high yield, reduce production Cost, fast response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Get 10g of Abamectin B1 (B1a content > 90%) and dissolve it in 40g of methylene chloride at room temperature, add 1.8g of allyl chloroformate and stir for 15min, slowly add 2.8g of triethylamine, 0.5g of triethylene The mixed solution of diamine and 2.0g dichloromethane was added in about 10 minutes. After the dropwise addition was completed, it was stirred at room temperature for 10 minutes. After the reaction was completed, the next oxidation reaction was continued. Add 2.4g DMSO, 2.3g triethylamine, and slowly drop Add 2.3g of phenyl phosphate dichloride, add dropwise in 5 minutes, and stir the reaction at room temperature for 30 minutes. After the reaction was completed, 20 g of water was added, 1 mol / L hydrochloric acid was added to adjust the pH to 2.5 and stirred, the phases were separated, and the organic phase was concentrated to obtain 10.36 g of a light yellow solid with a yield of 95%.
[0027] Add 30% NaOH solution to the aqueous layer to adjust the pH valu...
Embodiment 2
[0029] Take Abamectin B2 (B2a content>90%) 10g, be dissolved in 40g methylene chloride, after dissolving completely, add 2.4g allyl chloroformate and stir at room temperature for 15min, then slowly dropwise add 3.8g tributylamine, 0.002g The mixed solution of triethylenediamine was added dropwise for 5 minutes. After the addition was completed, it was stirred at -25°C for 60 minutes to complete the reaction. Continue to the next oxidation reaction, add 2.5g DMSO, 4.0g tributylamine, and slowly drop 2.4g phenyl phosphate The diacyl chloride was added dropwise in 5 minutes, and the reaction was stirred at room temperature for 30 minutes. After the reaction, 20 g of water was added, 1 mol / L hydrochloric acid was added to adjust the pH to 3.0, stirred, phases were separated, and the organic phase was concentrated to obtain 10.54 g of a light yellow solid with a yield of 97%.
[0030] Add 30% NaOH solution to the aqueous layer to adjust the pH value to >11, let stand, and separate ...
Embodiment 3
[0032] Take Abamectin B1 (B1a content > 90%) 10g, dissolve in 40g sec-butyl acetate, add 1.9g allyl chloroformate after dissolving completely, stir at room temperature for 15min, then slowly add 1.5g ethyldiisopropylamine dropwise , a mixed solution of 1.2g triethylamine and 0.4g tetramethylethylenediamine, the dropwise addition is completed in 5 minutes, after the addition is completed, stir at 0°C for 40 minutes to complete the reaction, continue the next oxidation reaction, add 2.4g DMSO, 3.0g Ethyldiisopropylamine, 1.8g of solid phosgene in 3ml of dichloromethane solution was slowly added dropwise, the dropwise addition was completed in 5 minutes, and the reaction was stirred at room temperature for 30 minutes. After the reaction, add 20 g of water, add 1 mol / L hydrochloric acid to adjust the pH to 3.0, stir, and separate phases. The organic phase was concentrated to obtain 10.15 g of a light yellow solid with a yield of 93%.
[0033] Add 30% NaOH solution to the aqueous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com