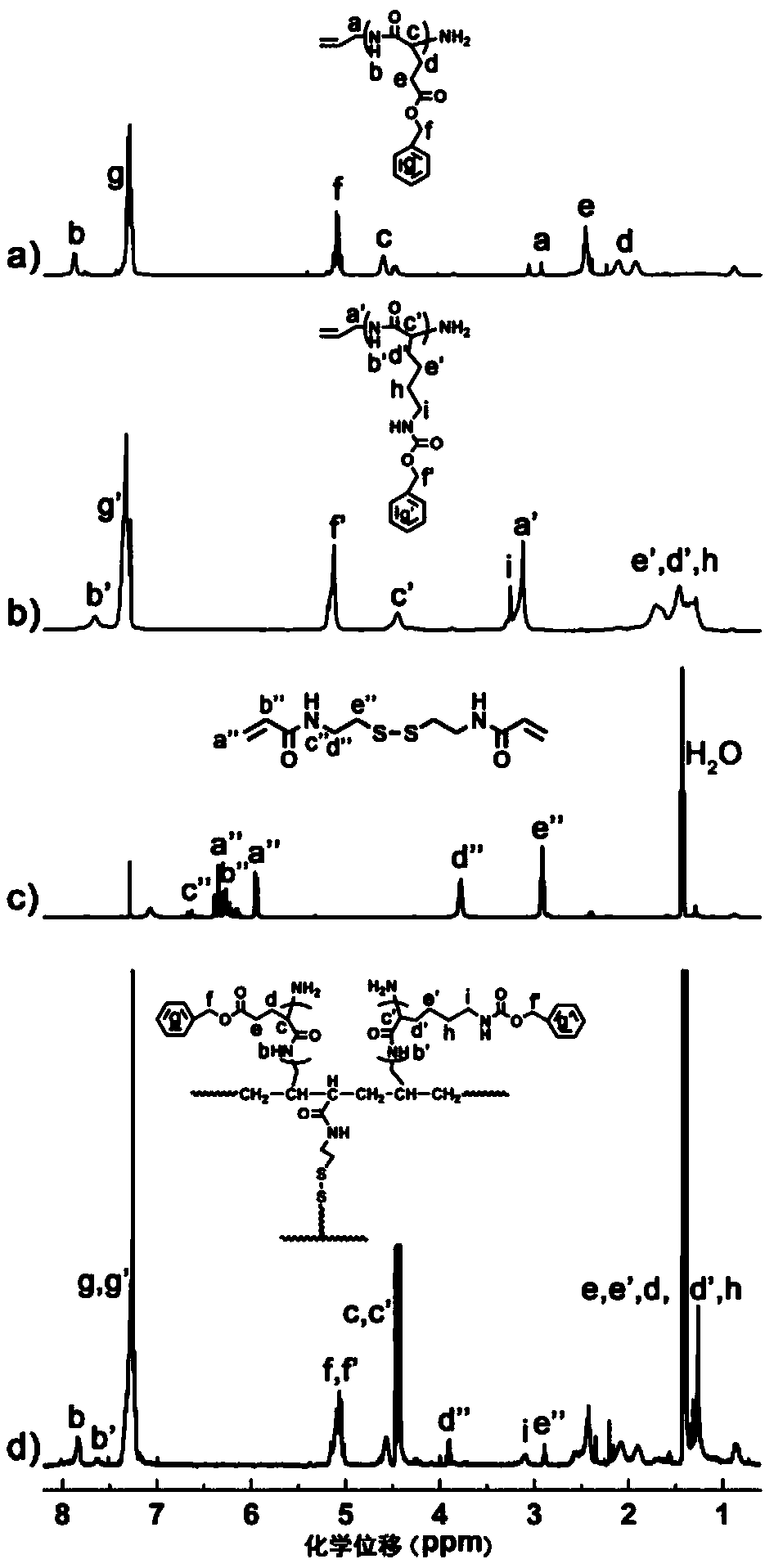
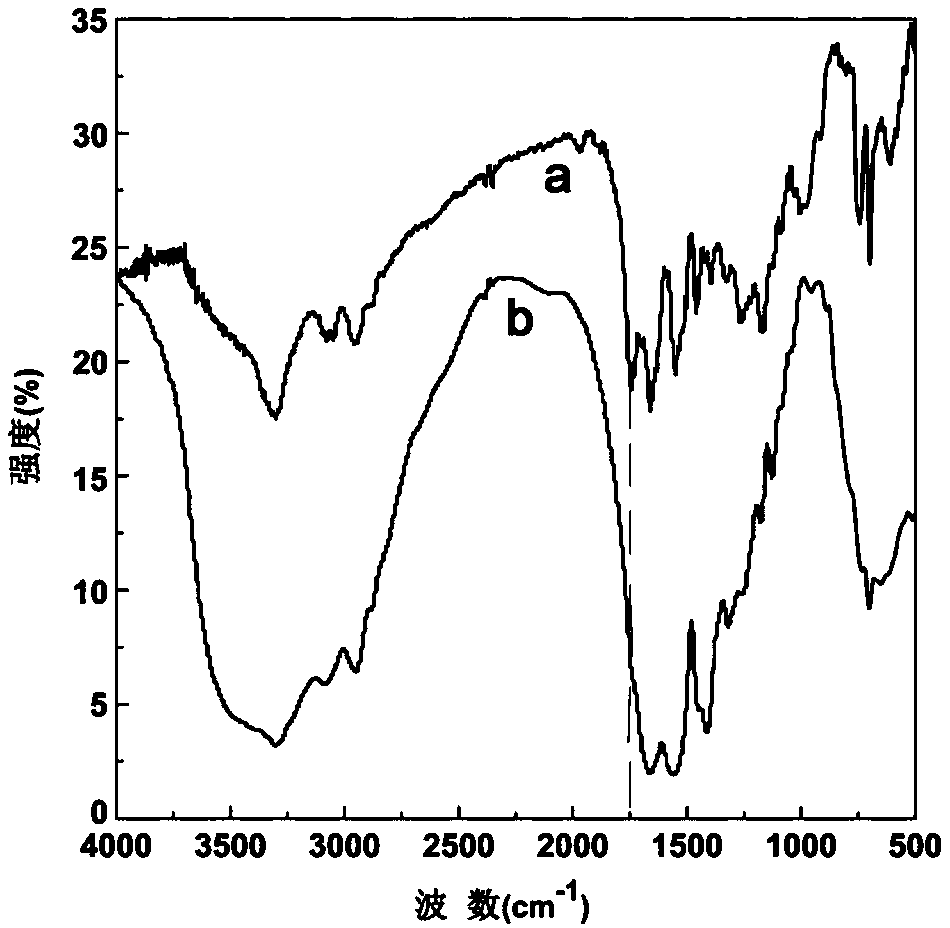
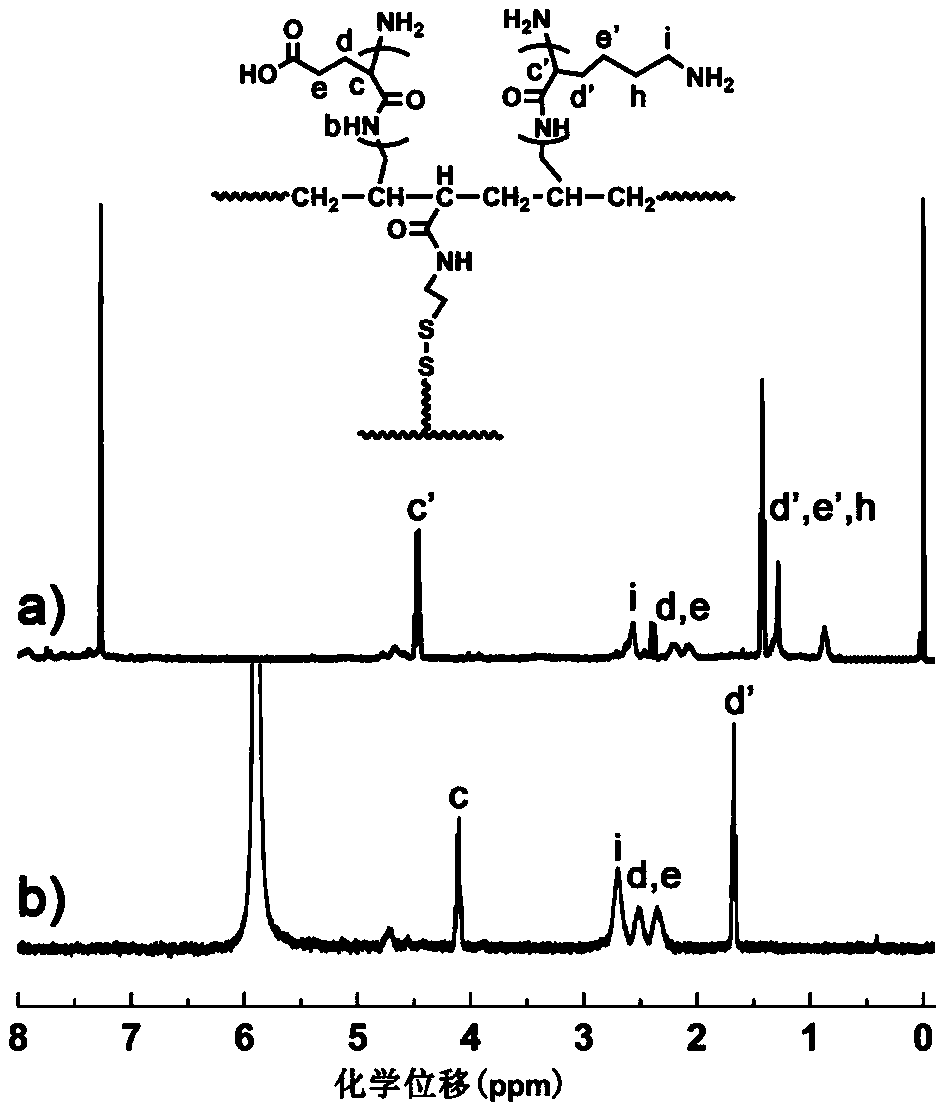
Charge reversal type polymeric micelles, charge reversal type polymeric drug-loading micelles and their preparation methods
A flip-type, polymer technology, used in pharmaceutical formulations, non-active ingredients medical preparations, emulsion delivery, etc., can solve the problems of tumor accumulation and poor permeability, poor blood circulation stability, and no environmental responsiveness. Achieve the effect of improving drug release efficiency, strong reduction responsiveness, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (1) Preparation of polybenzyloxycarbonyl lysine
[0054] Dissolve allylamine and benzyloxycarbonyllysine anhydride in DMF at a molar ratio of 1:15 to form a mixed solution with a total solute concentration of 0.50 g / mL, then react at 25°C for 12 hours, and then add to the reaction product Add diethyl ether for precipitation until the precipitated product no longer increases, and the resulting precipitated product is suction-filtered and dried at 60°C and 0.010MPa for 24 hours to obtain polybenzyloxycarbonyllysine.
[0055] (2) Preparation of polybenzyl glutamate
[0056] Dissolve allylamine and benzyl glutamate anhydride in DMF at a molar ratio of 1:20 to form a mixed solution with a total solute concentration of 0.50 g / mL, then react at 25°C for 12 hours, and then add Precipitate with diethyl ether until the precipitated product no longer increases, and the resulting precipitated product is suction-filtered and dried at 60°C and 0.010MPa for 24 hours to obtain polyben...
Embodiment 2
[0077] (1) Preparation of polybenzyloxycarbonyl lysine
[0078] Dissolve allylamine and benzyloxycarbonyllysine anhydride in CH at a molar ratio of 1:25 2 Cl 2 In this method, a mixed solution with a total solute concentration of 0.40g / mL was prepared, and then reacted at 30°C for 18 hours, and then added ether to the reaction product for precipitation until the precipitated product no longer increased. Dry at 60°C and 0.012MPa for 24 hours to obtain polybenzyloxycarbonyllysine.
[0079] (2) Preparation of polybenzyl glutamate
[0080] Dissolve allylamine and benzyl glutamate anhydride in CH at a molar ratio of 1:30 2 Cl 2 In this method, a mixed solution with a total solute concentration of 0.40g / mL was prepared, and then reacted at 30°C for 18 hours, and then added ether to the reaction product for precipitation until the precipitated product no longer increased. Dry at 60°C and 0.012MPa for 24 hours to obtain polybenzyl glutamate.
[0081] (3) Preparation of charge-re...
Embodiment 3
[0086] (1) Preparation of poly-tert-butoxycarbonyl lysine
[0087] Dissolve allylamine and tert-butoxycarbonyllysine cyclic anhydride in DMSO at a molar ratio of 1:35 to form a mixed solution with a total solute concentration of 0.30 g / mL, then react at 35°C for 24 hours, and then to the reaction product Diethyl ether was added to precipitate until the precipitated product no longer increased, and the obtained precipitated product was suction-filtered and dried at 60°C and 0.014MPa for 24 hours to obtain poly-tert-butoxycarbonyllysine.
[0088] (2) Preparation of poly tert-butyl glutamate
[0089] Dissolve allylamine and tert-butyl glutamate anhydride in THF at a molar ratio of 1:40 to form a mixed solution with a total solute concentration of 0.25 g / mL, then react at 25°C for 24 hours, and then add to the reaction product Add diethyl ether for precipitation until the precipitated product no longer increases, and the resulting precipitated product is suction-filtered and drie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com