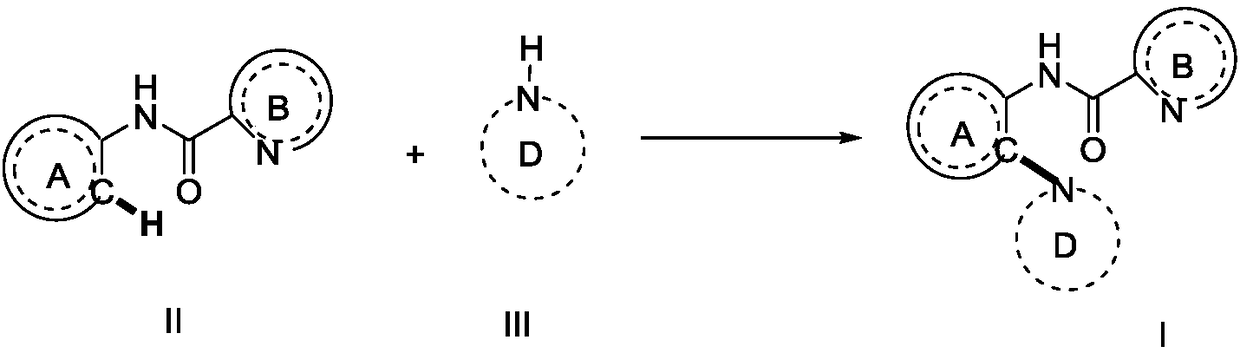
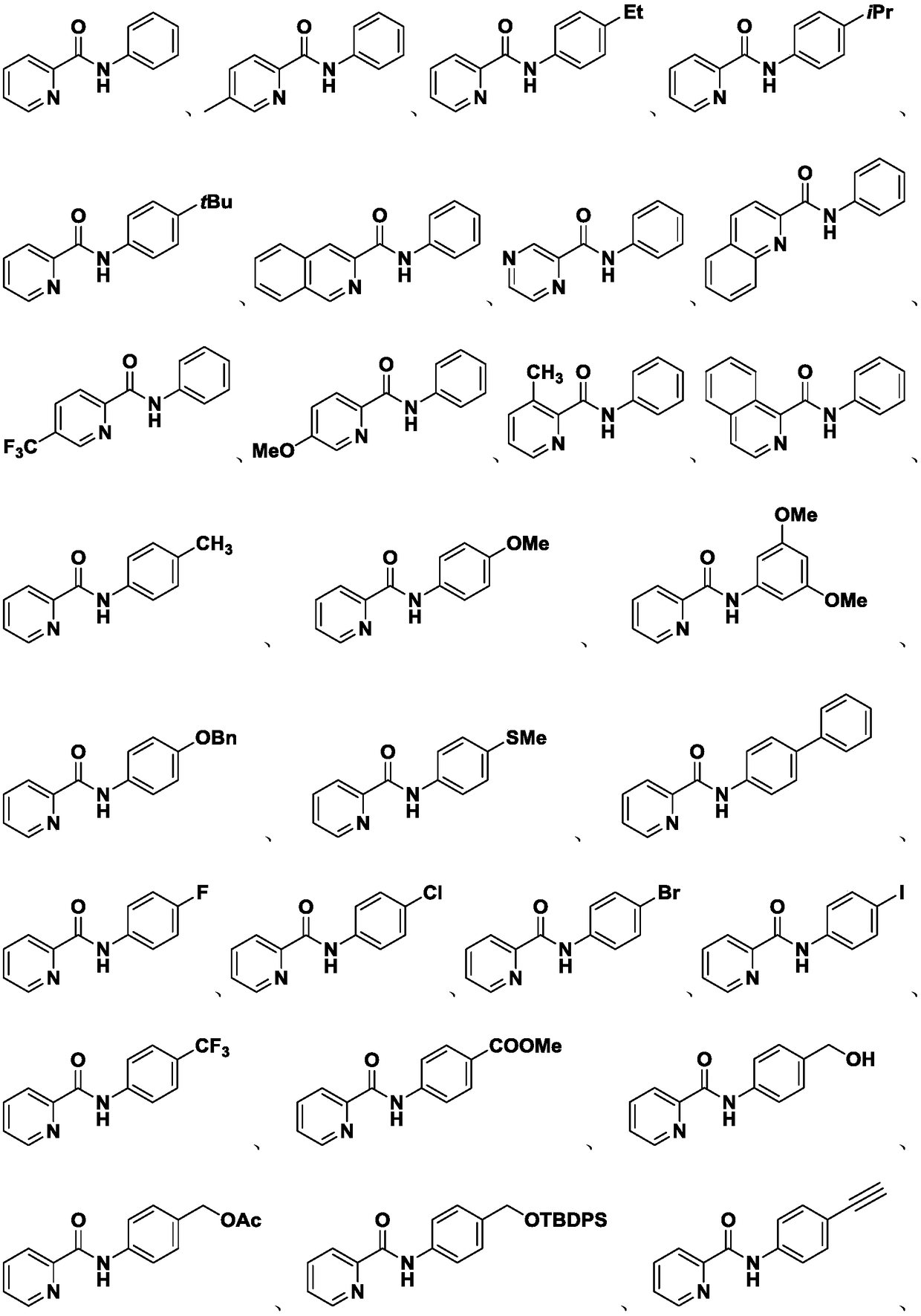
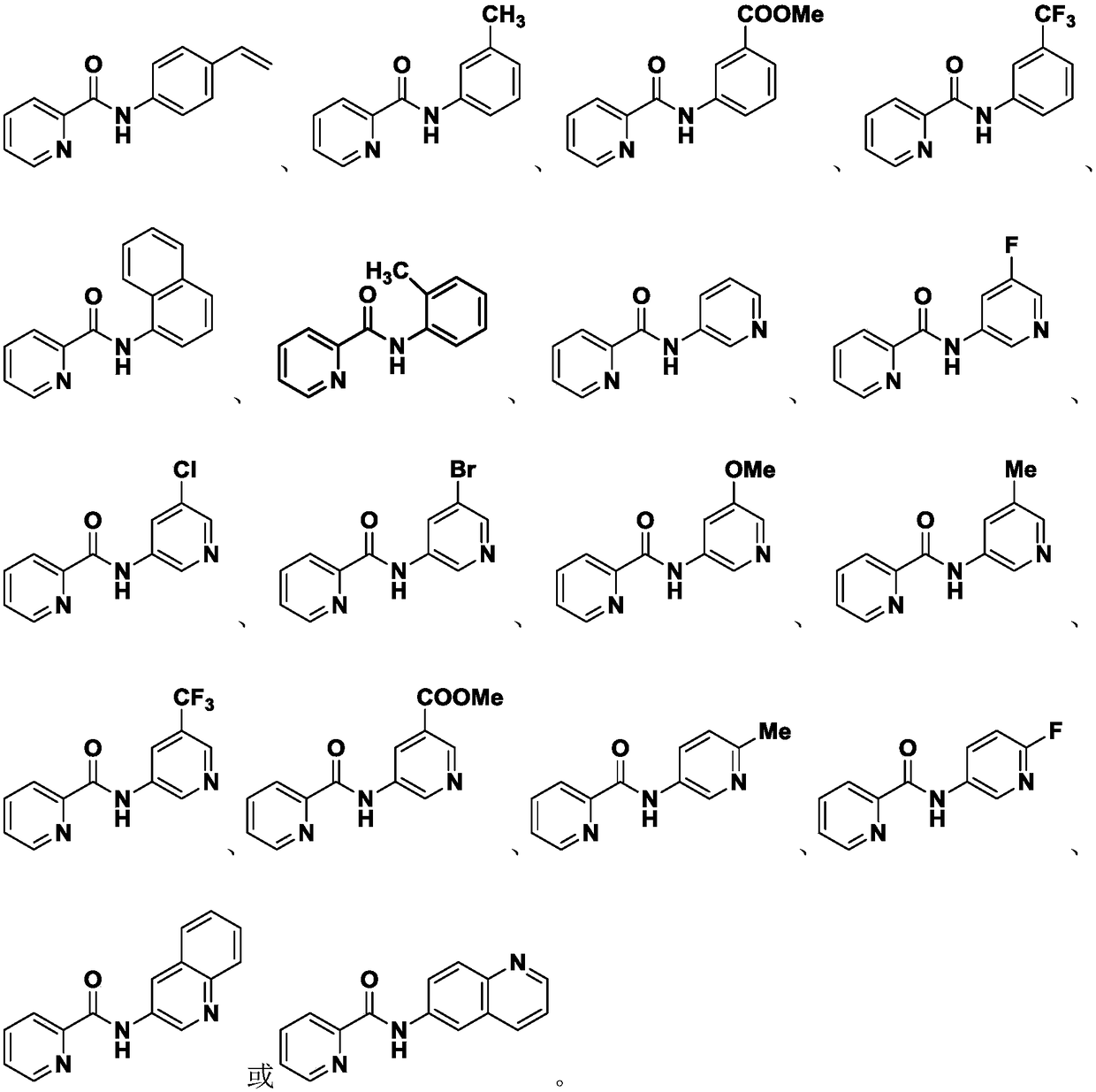
Preparation method of compound containing C (sp2)-N bonds
A compound, C3-C6 technology, used in the production of bulk chemicals, electrolysis processes, electrolysis components, etc., can solve problems such as poor selectivity and the use of large oxidants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0100]
[0101] Pyridinamide substrate 1a (39.6mg, 0.2mmol), morpholine (69.7mg, 0.8mmol), copper trifluoromethanesulfonate (7.3mg, 0.02mmol), potassium pivalate ( 56 mg, 0.4 mmol), tetrabutylammonium iodide (36.9 mg, 0.1 mmol) and acetonitrile (2 mL). Then add platinum sheets (1.5×1.0cm) to the cathode and anode respectively 2 ) electrode, pass a current of 3.0mA, and continue electrolysis at 27°C for 24h. After the reaction was completed, the solvent was suspended to dryness under reduced pressure, and then separated and purified by silica gel column chromatography to obtain a colorless solid 3a (48.7 mg, 0.172 mol, yield 86%, purity greater than 95%). 1 H NMR (400MHz, CDCl 3 ):δ11.06(s,1H),8.57-8.54(m,2H),8.21(d,J=7.6Hz,1H),7.79(td,J=7.6,1.6Hz,1H),7.37(ddd, J=7.6,4.8,0.8Hz,1H),7.13-6.99(m,3H),3.89(t,J=4.4Hz,4H),2.85(t,J=4.4Hz,4H). 13 C NMR (100MHz, CDCl 3 ): δ161.7, 150.3, 148.2, 141.8, 137.58, 132.9, 126.3, 125.1, 124.0, 122.2, 120.0, 119.4, 67.5, 52.3.
Embodiment 1-2
[0103]
[0104] With the above reaction conditions, starting from 1e (0.2 mmol), a colorless solid 3e (55.9 mg, yield 84%, purity greater than 95%) could be obtained. 1 H NMR (400MHz, CDCl 3 ): δ11.34(s,1H),9.27(s,1H),8.70(s,1H),8.67(dd,J=8.0,0.8Hz,1H),8.59(d,J=8.0Hz,1H) ,8.00(d,J=8.4Hz,1H),7.79-7.69(m,2H),7.24-7.09(m,3H),4.03(t,J=4.4Hz,4H),2.98(t,J=4.4 Hz,4H). 13 C NMR (100MHz, CDCl 3 ): δ162.4, 151.2, 144.2, 141.8, 136.1, 133.2, 131.1, 129.7, 129.0, 128.2, 127.7, 125.3, 123.9, 120.6, 120.0, 119.7, 67.7, 52.4.
Embodiment 1-3
[0106]
[0107] With the same reaction conditions as above, starting from 1f (0.2 mmol), a white solid 3f (48.3 mg, yield 85%, purity greater than 95%) can be obtained. 1 H NMR (400MHz, CDCl 3 ):δ10.91(s,1H),9.51(s,1H),8.80(d,J=2.4Hz,1H),8.64-8.63(m,1H),8.56(d,J=8.0Hz,1H) ,7.23-7.11(m,3H),3.95(t,J=4.4Hz,4H),2.94(t,J=4.0Hz,4H). 13 C NMR (100MHz, CDCl 3 ): δ160.5, 147.4, 145.1, 144.7, 142.7, 141.8, 132.6, 125.5, 124.6, 120.4, 119.6, 67.6, 52.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com