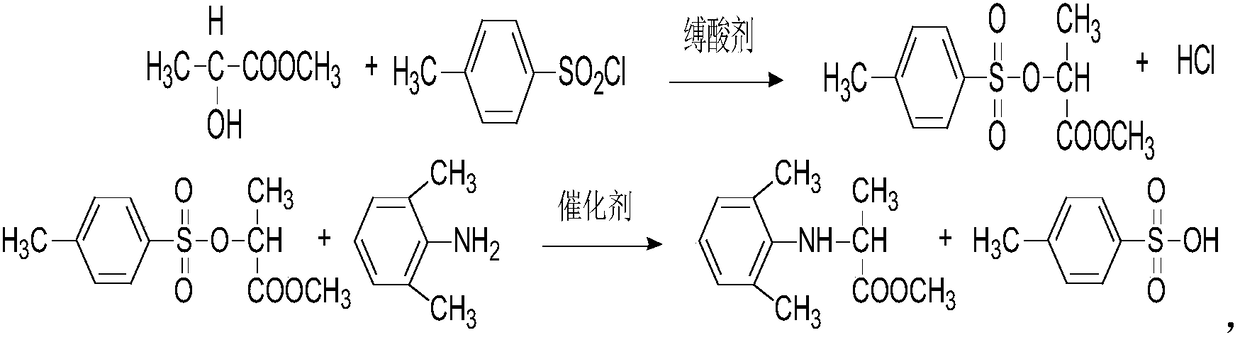
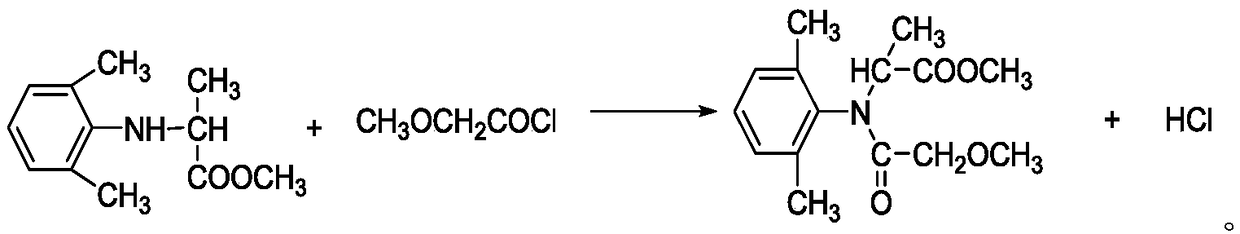
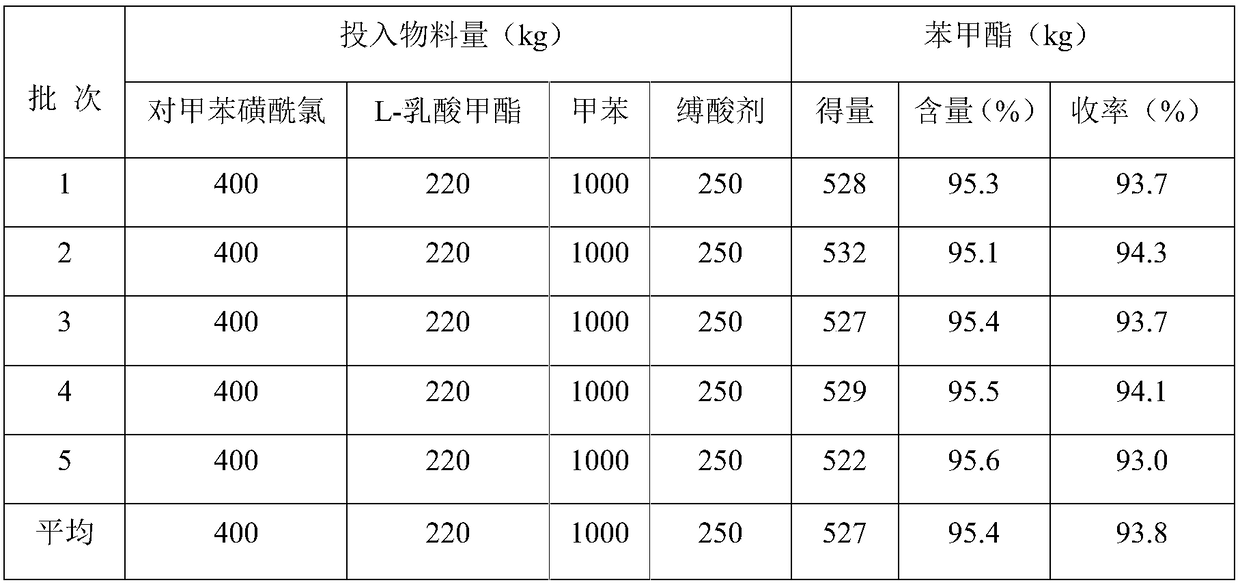
Synthesis method of optically active metalaxyl
A synthetic method, the technology of metalaxyl, applied in the field of synthesis of optically active metalaxyl, can solve the problems of low total yield, environmental pollution, low yield, etc., and achieve the reduction of three wastes, product yield and yield High and simplified operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of synthetic method of optically active metalaxyl
[0033] Optically active metalaxyl, synthesized by the following method:
[0034] Step S10, add 200kg of methanol and 190kg (2kmol) of chloroacetic acid into a 3000L reaction kettle, start stirring, and heat up until the materials are basically dissolved, then add 790kg (4.1kmol) of 28% sodium methoxide, heat up to 58°C, and keep warm for 0.5 hour, the reaction is over, methanol is recovered under reduced pressure, the temperature is at 80°C, and the vacuum degree is 0.098Mpa;
[0035] Step S20, after the decompression is completed, the temperature is lowered to 35°C, 1800kg of toluene is added, 94.4kg (0.68kmol) of phosphorus trichloride is added dropwise at 40°C, the addition is completed in 35 minutes, and the temperature is kept at 50°C for 1.2 hours;
[0036] Step S30, after the heat preservation is completed, the tail gas absorption device is turned on, and 393kg (95%, 1.8kmol) of D-N-(2,6-dimethylphenyl)a...
Embodiment 2
[0042] A kind of synthetic method of optically active metalaxyl
[0043] Optically active metalaxyl, synthesized by the following method:
[0044] Step S10, add 300kg of methanol and 190kg (2kmol) of chloroacetic acid into a 3000L reaction kettle, start stirring, and heat up until the materials are basically dissolved, then add 790kg (4.1kmol) of 28% sodium methoxide, heat up to 65°C, and keep warm for 1 hour, the reaction is over, methanol is recovered under reduced pressure, the temperature is 75°C, and the vacuum is 0.098Mpa;
[0045] Step S20, after the decompression is completed, the temperature is lowered to 40°C, 1300kg of toluene is added, 105.6kg (0.76kmol) of phosphorus trichloride is added dropwise at 45°C, the addition is completed in 45 minutes, and the temperature is kept at 40°C for 0.8 hours;
[0046] Step S30, after the heat preservation is completed, the tail gas absorption device is turned on, and 436 kg (95%, 2.0 kmol) of D-N-(2,6-dimethylphenyl)alanine me...
Embodiment 3
[0052] A kind of synthetic method of optically active metalaxyl
[0053] Optically active metalaxyl, synthesized by the following method:
[0054] Step S10, add 300kg of methanol and 190kg (2kmol) of chloroacetic acid into a 3000L reaction kettle, start stirring, heat up until the materials are basically dissolved, add 732kg (3.8kmol) of 28% sodium methoxide, heat up to 60°C, and keep warm for 1.2 hour, the reaction is over, methanol is recovered under reduced pressure, the temperature is at 72°C, and the vacuum degree is 0.098Mpa;
[0055] Step S20, after the decompression is completed, the temperature is lowered to 38°C, 1600kg of toluene is added, 83.3kg (0.6kmol) of phosphorus trichloride is added dropwise at 43°C, and the addition is completed in 25 minutes, and the temperature is kept at 60°C for 0.5 hours;
[0056] Step S30, after the heat preservation is completed, the tail gas absorption device is turned on, and 427 kg (95%, 1.96 kmol) of D-N-(2,6-dimethylphenyl)alan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com