A torasemide for injection and preparation process thereof
A torasemide preparation technology, applied in the field of torasemide preparations for injection and its preparation process, can solve the problem of poor solubility and stability of torasemide freeze-drying, poor stability, long reconstitution time, etc. problems, achieve excellent resolubilization performance, considerable economic and social benefits, and complete moisture drying
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 prescription 1 and preparation technology
[0034] Prescription 1:
[0035] prescription
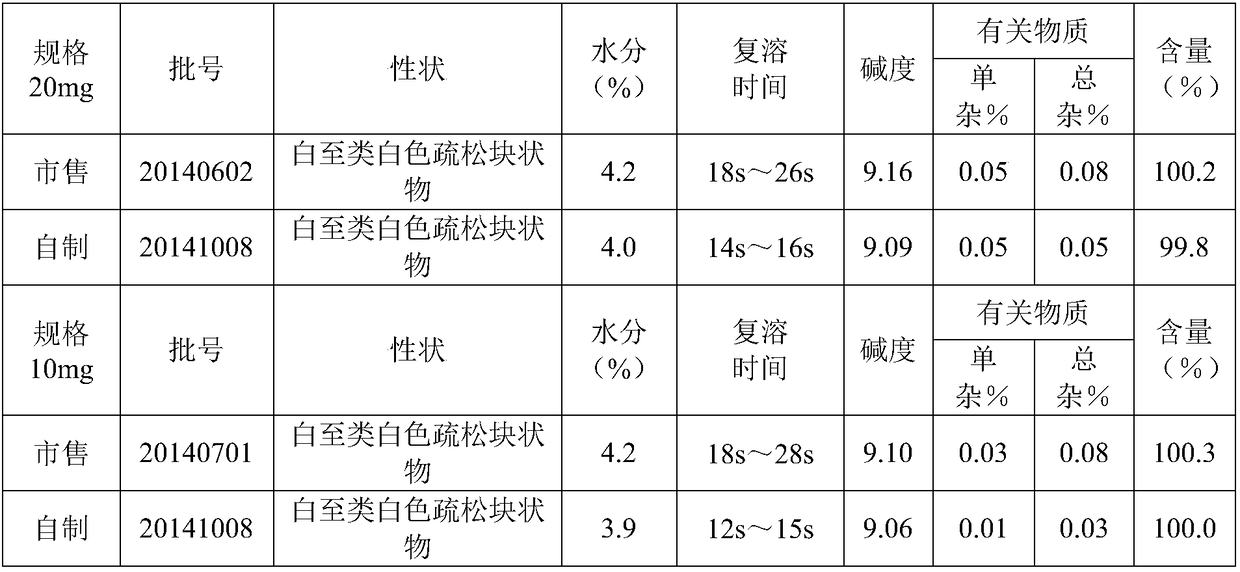
Specification 20mg
20g
20g
10% sodium hydroxide solution
Appropriate amount
10% hydrochloric acid solution
Appropriate amount
160g
water for injection
up to 2kg
[0036] Preparation Process:
[0037] (1) Take the raw and auxiliary materials according to the prescription, prepare a pH regulator (10% sodium hydroxide solution, 10% hydrochloric acid solution), and set aside;
[0038] (2) Weigh 70% water for injection, add sodium bicarbonate and stir to dissolve, then add torasemide and stir to make it fully suspended;
[0039] (3) Stir and slowly add an appropriate amount of 10% sodium hydroxide solution until the main ingredient is completely dissolved, and measure the pH value of the solution;
[0040] (4) Stir and slowly add an appropriate amount of...
Embodiment 2
[0044] Embodiment 2 prescription 2 and preparation technology
[0045] Prescription 2:
[0046] prescription
[0047] Preparation process: According to the above prescription amount, it is prepared by the same process as in Example 1, named 20141008 (10 mg).
Embodiment 3
[0048] Embodiment 3 prescription 3 and preparation technology
[0049] Prescription 3:
[0050] prescription
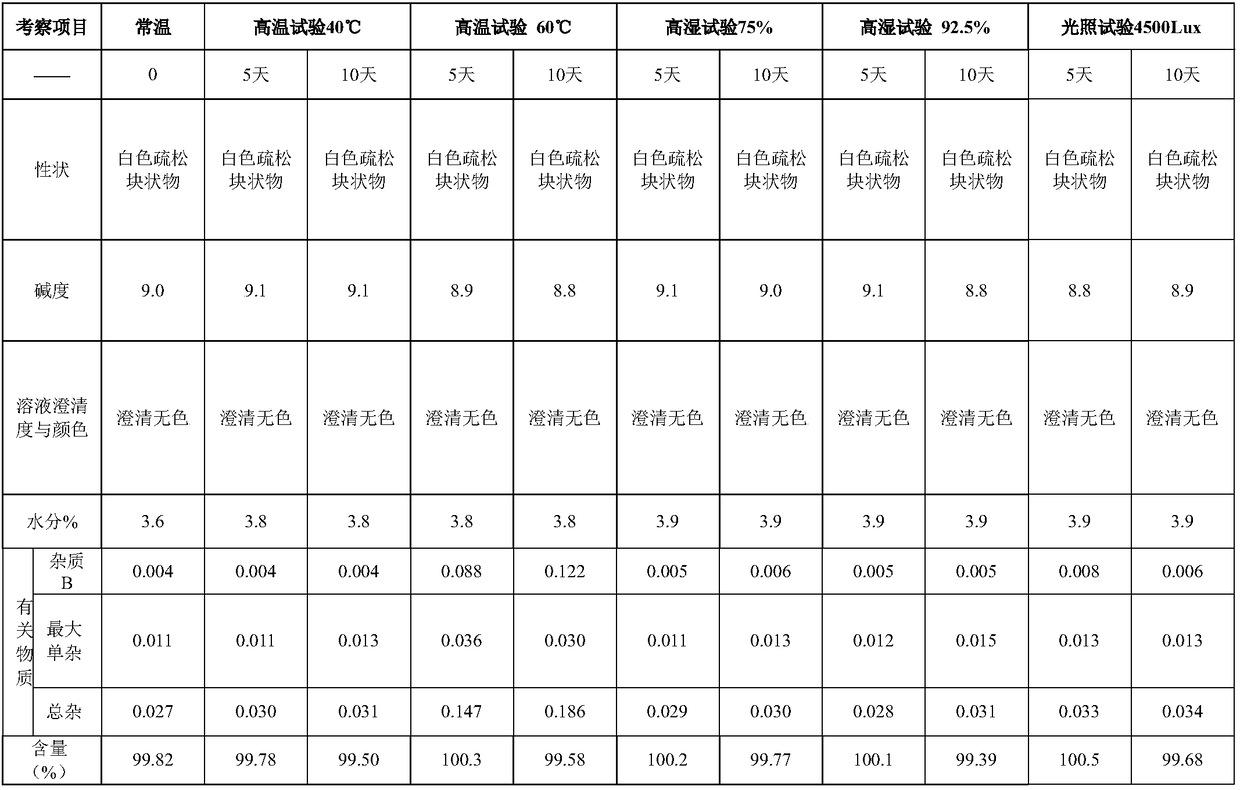
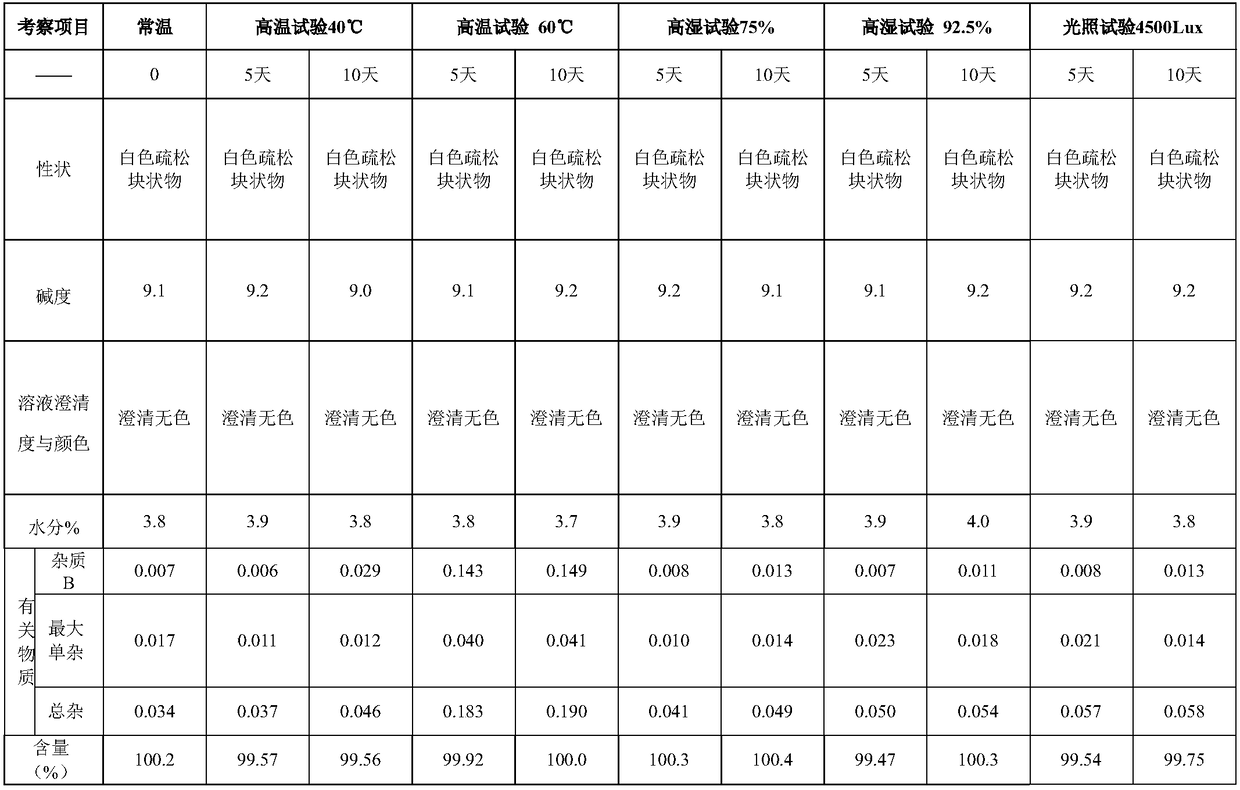
Specification 20mg
100g
100g
10% sodium hydroxide solution
Appropriate amount
10% hydrochloric acid solution
Appropriate amount
800g
water for injection
up to 10kg
[0051] Preparation Process:
[0052] (1) cooling the water for injection to below 45°C for subsequent use; weighing torasemide, sodium bicarbonate, and mannitol successively according to the prescription amount for subsequent use; preparing 10% sodium hydroxide solution and 10% hydrochloric acid solution for subsequent use;
[0053] (2) Weigh 70% of the prescribed amount of water for injection (below 45°C), add the prescribed amount of sodium bicarbonate, and stir until completely dissolved;
[0054](3) Add the prescribed amount of torasemide and stir until fully suspended;
[0055] (4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com