Compound B2 as histone methyltransferase NSD3 activity inhibitor and application thereof
A technology of methyltransferase and activity inhibitor, which is applied in the field of medicine and can solve the problems of less research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
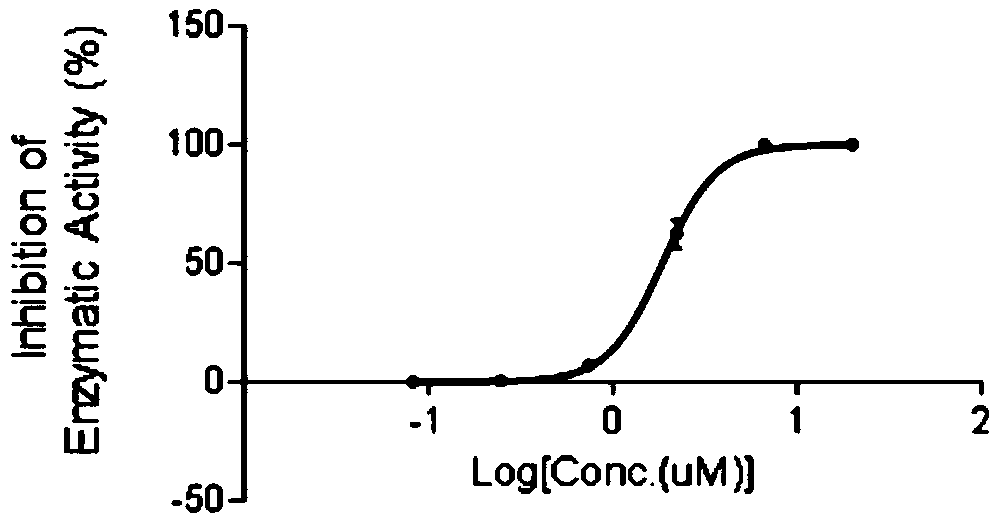
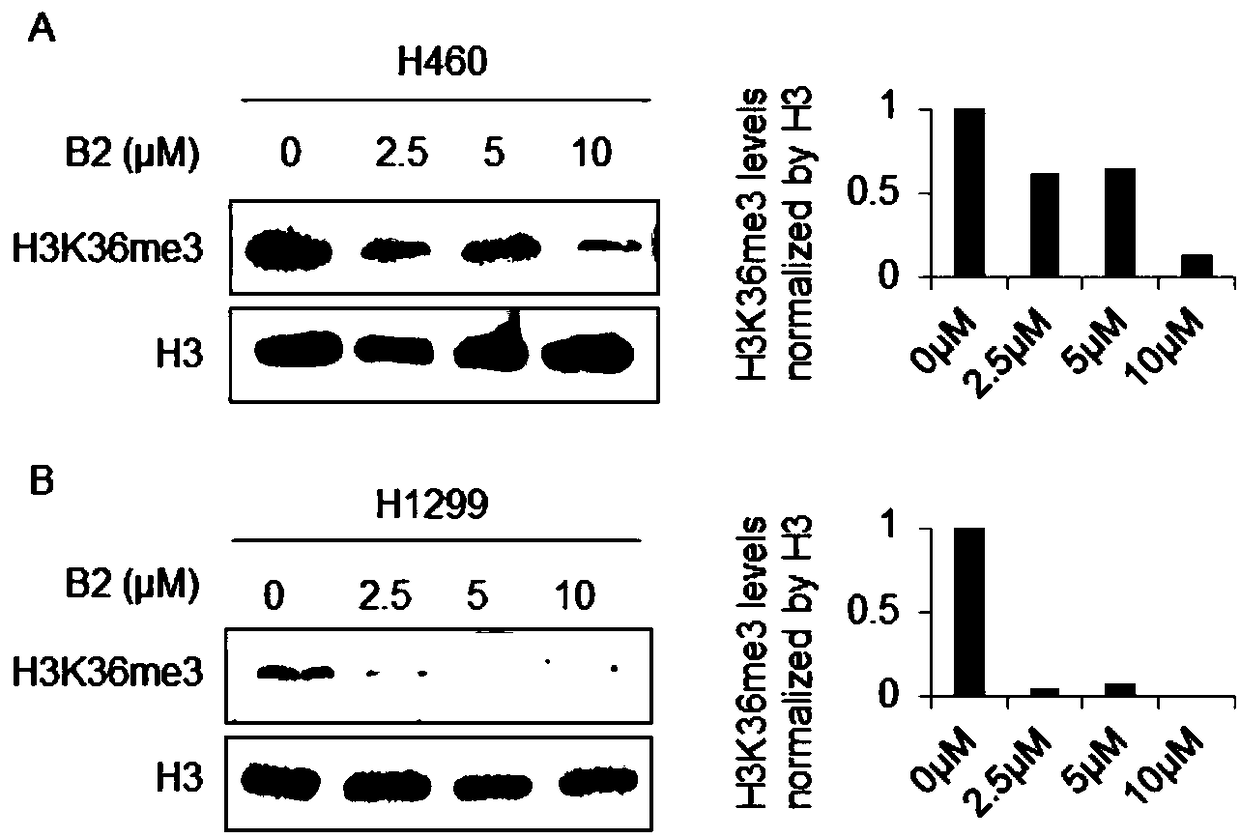
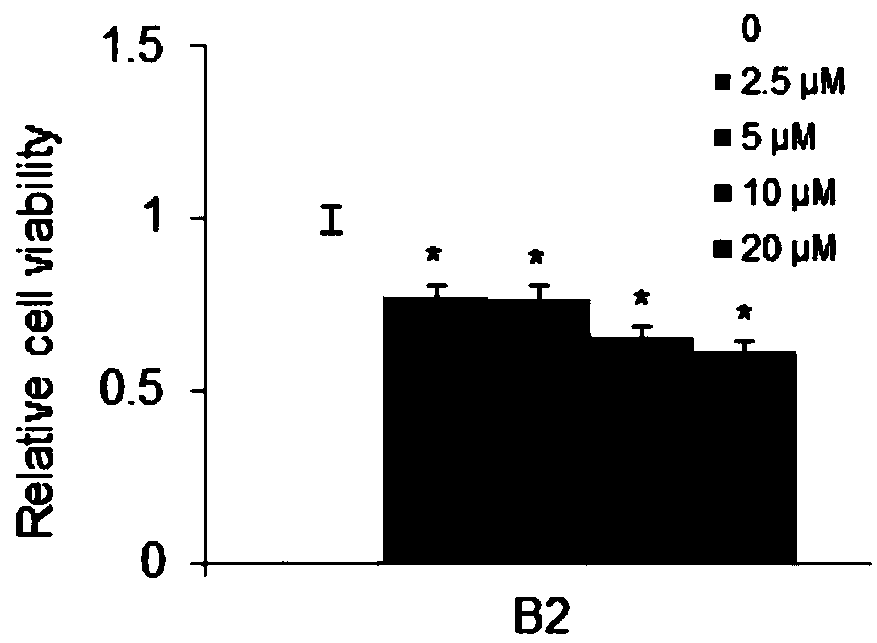
[0018] In order to confirm the antitumor effect of the compound of the present invention, the present invention will be further described below in conjunction with the accompanying drawings and specific examples.
[0019] 1. Experimental method
[0020] 1.1 Receptor-based virtual screening
[0021] first use The protein preparation module Protein Preparation Wizard in the software package processes the crystal structure of NSD3 (PDB: 4YZ8). The ChemDiv database was preprocessed with Discovery Studio 2.5, including deduplication, removal of salt ions and inorganic substances, and structural standardization. use The LigPrep module in 9.0 generates probable ionization states and tautomers of compounds at pH = 7.4.
[0022] Before using the molecular docking method for virtual screening, it is first necessary to verify the effectiveness of the Glide docking method used, define the active site of NSD3, and set the center of mass of the ligand molecule S-adenosyl methionine (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com