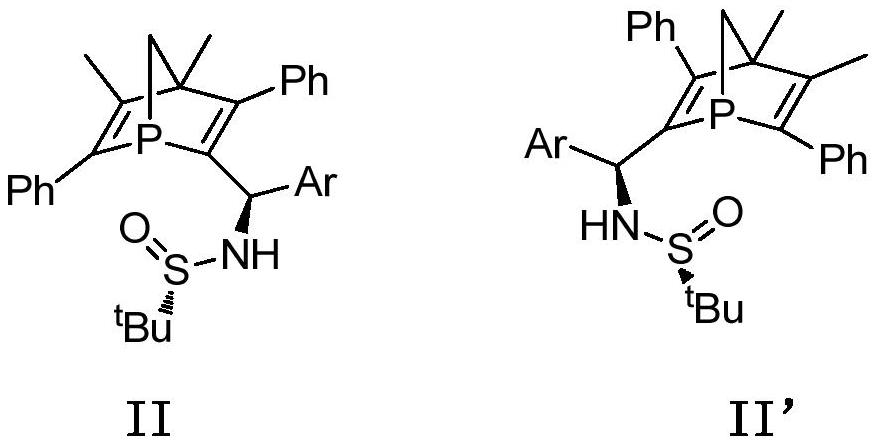
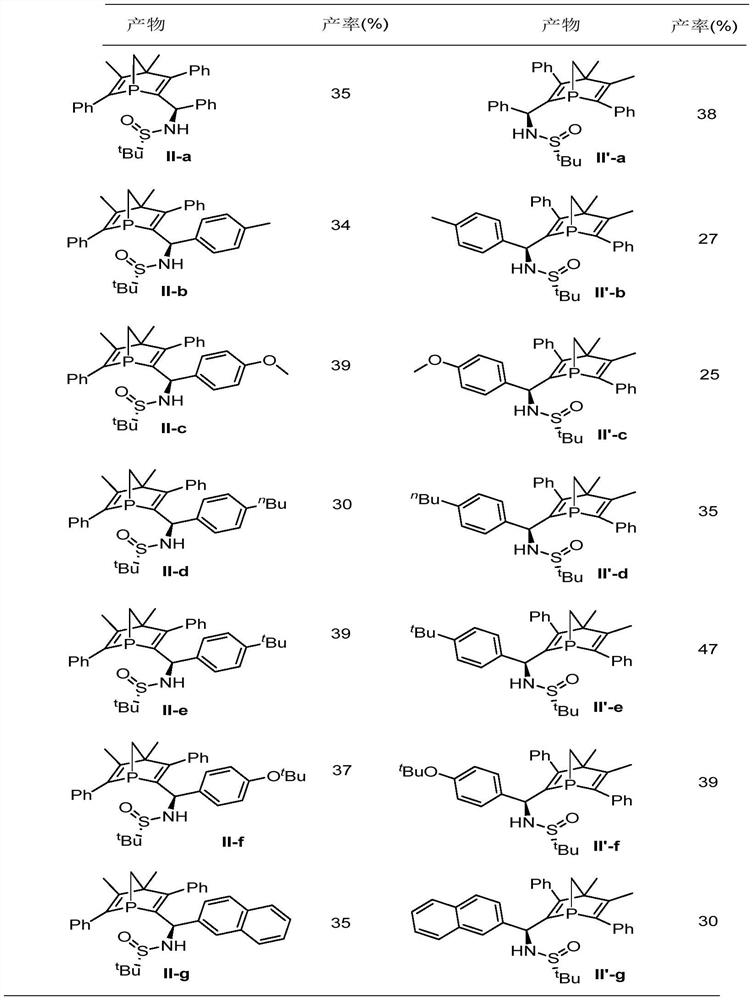
Chiral 1-phosphanorbornadiene derivative and its synthesis method
A norbornan and phosphatic technology is applied in the field of 1-phospha norbornadiene compounds and their synthesis, and achieves the effects of high yield, simple synthesis method and broad commercial application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]
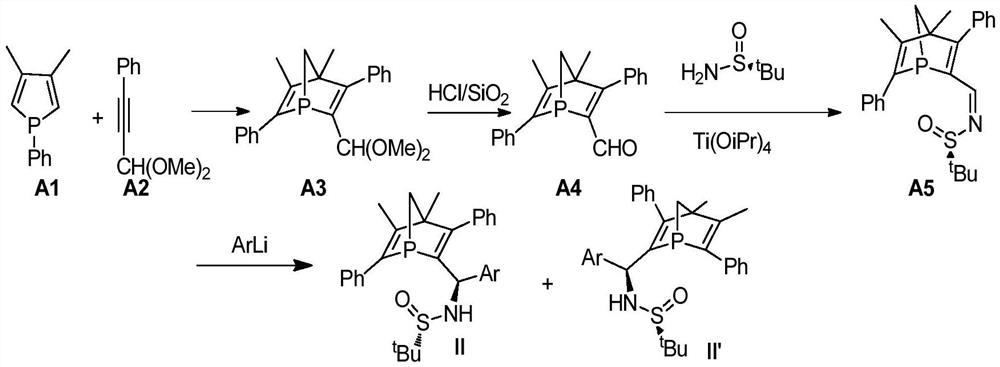
[0022] (1) in N 2 Under ambient conditions, add 40mL of methanol, phenylpropynaldehyde (20mmol), and trimethyl orthoformate (40mmol) into a 100mL Schlenk bottle, then add the catalyst p-toluenesulfonic acid (10mol%), and transfer to a 65°C oil bath to reflux for 10h. After the reaction was complete, a small amount of solid NaOH was added, and stirring was continued for 20 min. After rotary evaporation under reduced pressure, the compound A2 was obtained after passing through a neutral alumina drying column with a yield of 93%.
[0023]
[0024] (2) in N 2 At ambient, 5 mL of toluene, compound A2 (5.6 mmol), phosphole (5.3 mmol) were added to a 75 mL Schlenk bottle. React in an oil bath at 140°C for 2h. After passing through a neutral alumina column (PE:DCM=10:1), compound A3 was obtained. Yield 80%.
[0025] (3) in N 2 Under ambient conditions, add 20 mL of dichloromethane, compound A3, hydrochloric acid, and silicon dioxide into a 100 mL Schlenk bottle, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com