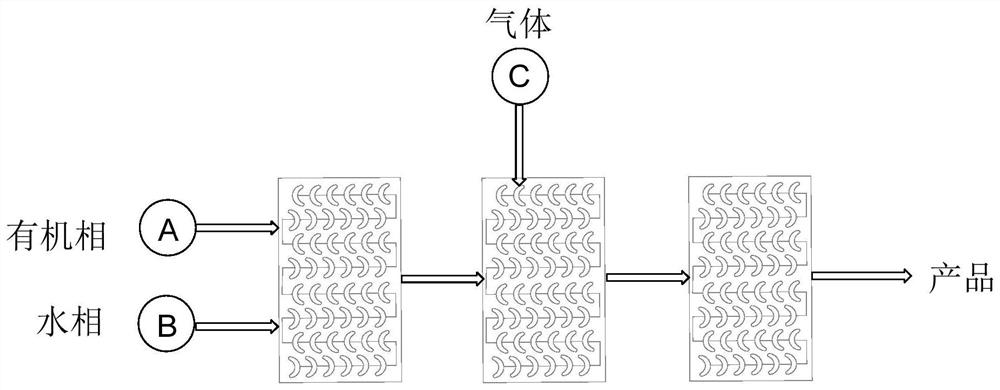
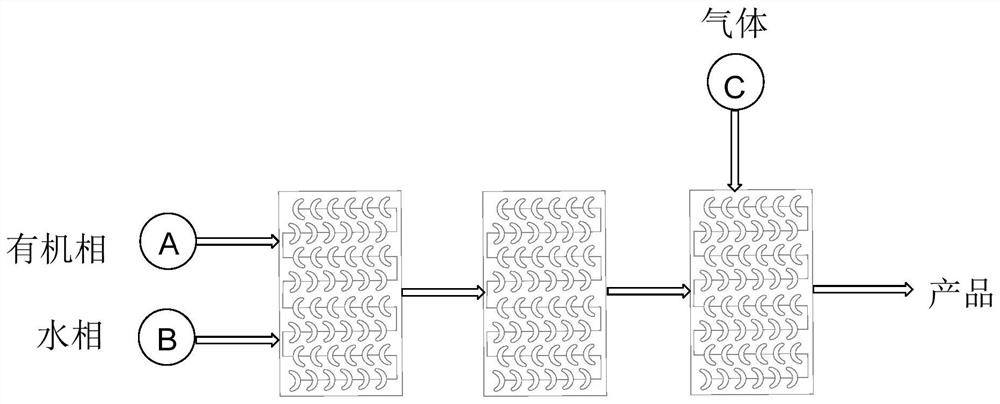
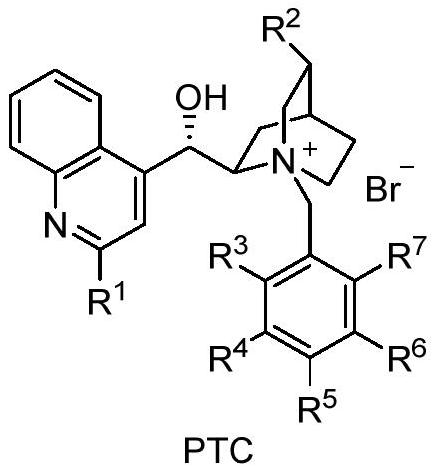
A method for the continuous preparation of chiral α-hydroxy-β-dicarbonyl compounds by visible light-catalyzed molecular oxygen oxidation via a microreactor
A technology of dicarbonyl compounds and microreactors, which is applied in the field of flow chemistry to achieve the effect of mild reaction conditions and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] Take by weighing 0.05mmol 1-indanone-2-carboxylate adamantyl (Ia-1), 10% PTC-2, 1% tetraphenylporphyrin, put into 10ml test tube, add 5ml toluene, solid dissolves completely, and is recorded as A bottle; another 0.5% potassium carbonate solution is prepared, which is recorded as bottle b, at 0°C, the light source is white light, the organic phase in bottle a is pumped from pump A at a rate of 0.3ml / min, and the alkaline solution in bottle b is pumped from pump B at a rate of The flow rate of oxygen into C is 0.3ml / min, the flow rate of oxygen is 1ml / min, and the residence time is about 1.5min. The conversion rate of the reaction reaches 99.9%, the selectivity of α-hydroxylation product is more than 95%, and the ee value is 84%.
Embodiment 2
[0031]
[0032] Take by weighing 0.05mmol 1-indanone-2-carboxylate adamantyl (Ia-1), 10% PTC-2, 1% tetraphenylporphyrin, put into 10ml test tube, add 5ml toluene, solid dissolves completely, and is recorded as bottle a; another 0.5% potassium carbonate solution is prepared, which is recorded as bottle b, at 0°C, the light source is white light, the organic phase in bottle a is pumped from pump A at a rate of 1.5ml / min, and the alkaline solution in bottle b is pumped from pump B at a rate of The flow rate of oxygen into C is 1.5ml / min, the flow rate of oxygen is 2ml / min, and the residence time is about 1.5min. The conversion rate of the reaction reaches 99.9%, the selectivity of α-hydroxylation product is more than 95%, and the ee value is 80%.
Embodiment 3
[0034]
[0035] Take by weighing 0.05mmol 1-indanone-2-carboxylate adamantyl (Ia-1), 10% PTC-3, 1% tetraphenylporphyrin, put into 10ml test tube, add 5ml toluene, solid dissolves completely, and is recorded as bottle a; another 0.5% potassium carbonate solution is prepared, which is recorded as bottle b, at 0°C, the light source is white light, the organic phase in bottle a is pumped from pump A at a rate of 1.5ml / min, and the alkaline solution in bottle b is pumped from pump B at a rate of The flow rate of oxygen into C is 1.5ml / min, the flow rate of oxygen is 2ml / min, and the residence time is about 1.5min. The conversion rate of the reaction reaches 99.9%, the selectivity of α-hydroxylation product is more than 95%, and the ee value is 84%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com