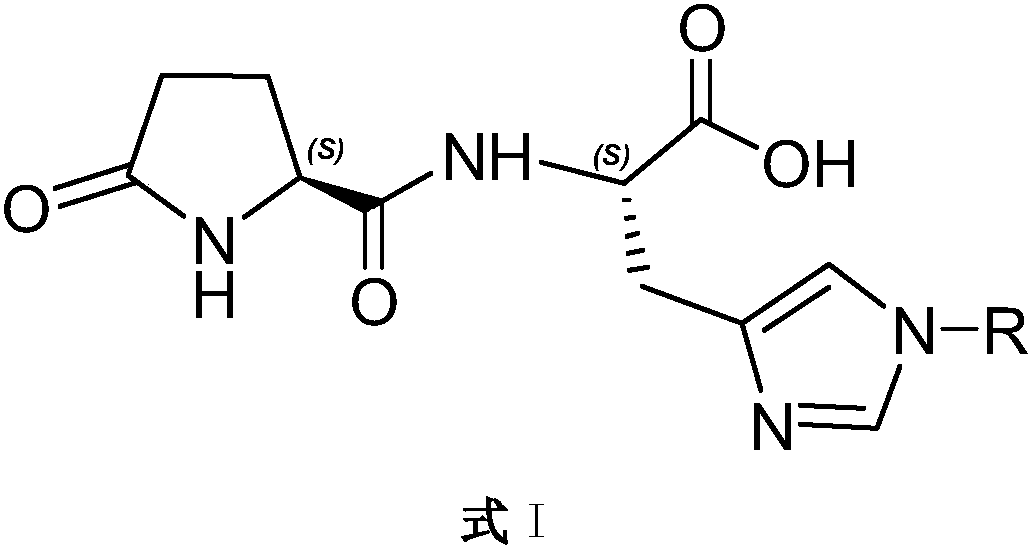
Dipeptide for synthesizing relin drugs
A drug, Relin's technology, applied in the field of peptide synthesis, can solve problems that affect product quality, drug safety, difficulty in complete removal, and product yield that cannot be effectively improved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The preparation of embodiment 1, H-Pyr-His (Fmoc)-OH
[0054] Add anhydrous methanol (1.35L) to a 5L two-neck flask equipped with electromagnetic stirring, and add SOCl dropwise under ice-cooling 2 (78.1ml, 1100.0mmol), control the reaction temperature below -10°C, maintain the low temperature reaction for 1h, then add histidine (His) (77.5g, 500.0mmol) at one time, and heat to reflux for 1h. After the reaction, concentrate under reduced pressure to remove methanol and SOCl 2 , to obtain white solid H-His-OMe·2HCl (110.9 g, yield 91.61%). Ms=169.99(M+H + ).
[0055] Pyroglutamic acid (12.9g, 100.0mmol) and 200ml DMF were added to a 1.5L flask, HBTU (37.9g, 100.0mol) was added under ice cooling, the pH was adjusted to about 8 with NMM, and stirred for 30min under ice cooling. Add H-His-OMe·2HCl (24.2g, 100.0mol) and 600ml DMF to another single-necked bottle, adjust the pH to about 8 with NMM, and pre-cool. Add the latter after the activation of the carboxyl componen...
Embodiment 2
[0058] The preparation of embodiment 2, H-Pyr-His (Boc)-OH
[0059] Add anhydrous methanol (1.35L) to a 5L two-neck flask equipped with electromagnetic stirring, and add SOCl dropwise under ice-cooling 2 (78.1ml, 1100.0mmol), control the reaction temperature below -10°C, maintain the low temperature reaction for 1h, then add histidine (His) (77.5g, 500.0mmol) at one time, and heat to reflux for 1h. After the reaction, concentrate under reduced pressure to remove methanol and SOCl 2 , to obtain white solid H-His-OMe·2HCl (110.9 g, yield 91.61%).
[0060] Pyroglutamic acid (12.9g, 100.0mmol) and 200ml DMF were added to a 1.5L flask, HBTU (37.9g, 100.0mol) was added under ice cooling, the pH was adjusted to about 8 with NMM, and stirred for 30min under ice cooling. Add H-His-OMe·2HCl (24.2g, 100.0mol) and 600ml DMF to another single-necked bottle, adjust the pH to about 8 with NMM, and pre-cool. Add the latter after the activation of the carboxyl component is completed, adjust...
Embodiment 3
[0063] The preparation of embodiment 3, H-Pyr-His(Trt)-OH
[0064] Add anhydrous methanol (1.35L) to a 5L two-neck flask equipped with electromagnetic stirring, and add SOCl dropwise under ice-cooling 2 (78.1ml, 1100.0mmol), control the reaction temperature below -10°C, maintain the low temperature reaction for 1h, then add H-His(Trt)-OH (198.7g, 500.0mmol) in one go, and heat to reflux for 1h. After the reaction, concentrate under reduced pressure to remove methanol and SOCl 2 , to obtain white solid H-His(Trt)-OMe·2HCl (206.6 g, yield 85.2%).
[0065] Pyroglutamic acid (5.3g, 41.1mmol) and 70ml DMF were added to a 500ml flask, HBTU (15.6g, 41.1.0mol) was added under ice cooling, the pH was adjusted to about 8 with NMM, and stirred for 30min under ice cooling. Add H-His(Trt)-OMe·2HCl (19.9g, 41.1mol) and 200ml DMF to another single-necked bottle, adjust the pH to about 8 with NMM, and pre-cool. Add the latter after the activation of the carboxyl component is completed, adj...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com