Small molecule regulators of steroid receptor coactivators and methods of use thereof
An unsubstituted, compound technology applied in the field of small molecule modulators of steroid receptor coactivators and their use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0386] Embodiment 1: the synthesis of compound
[0387] All chemicals were purchased from Sigma-Aldrich (Milwaukee, WI) or Alfa Aesar (Ward Hill, MA) unless otherwise noted. All other solvents and reagents were used as obtained without further purification. By Varian 400-MR spectrometer (Palo Alto, CA) on 1 H-NMR to characterize the compound. Chemical shifts (δ) are referenced to solvent signals [ 1 H NMR: DMSO-d6(2.50)] are given in ppm. UV-Vis measurements were performed in 10 × 10 mm quartz cuvettes using a Cary 60 UV-Vis spectrometer. Flash chromatography was performed on a Teledyne ISCO CombiFlash Rf200. ESI mass spectra were measured on an Agilent mass spectrometer (6130 single quadrupole).
[0388] Compounds as described herein are prepared according to the methods described below. In some cases, the synthesized compounds existed as mixtures of tautomers.
[0389] Synthesis of compound SI-3:
[0390]
[0391] To a solution of 2-chloro-lH-benzo[d]imidazole (9...
Embodiment 2
[0429] Example 2: Small Molecule Modulators of Steroid Receptor Coactivators
[0430] Described herein is a new class of small molecule modulators (ie, inhibitors and stimulators) of steroid receptor coactivators that are useful in the treatment of neurological and metabolic disorders, inflammatory diseases, and cancer.
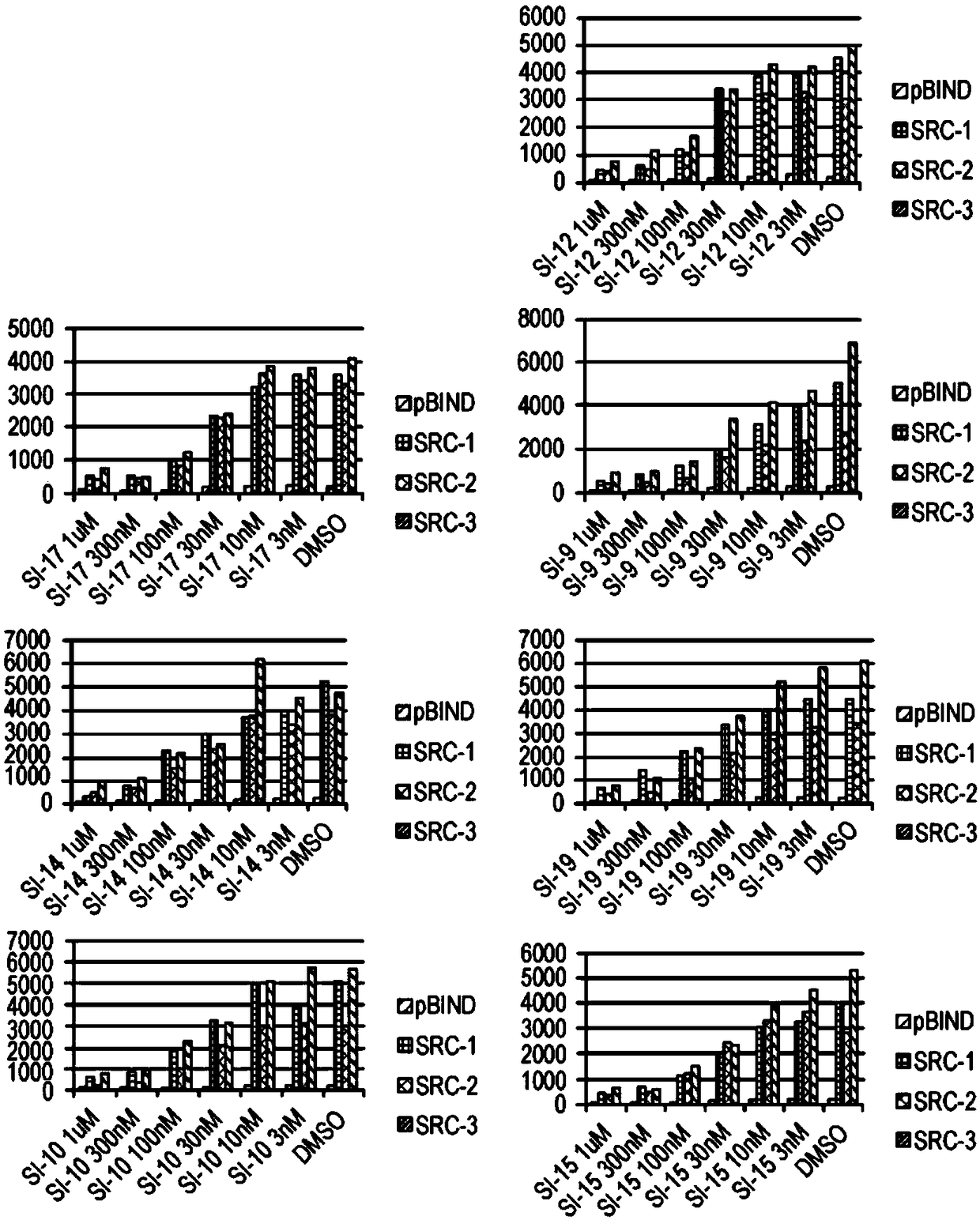
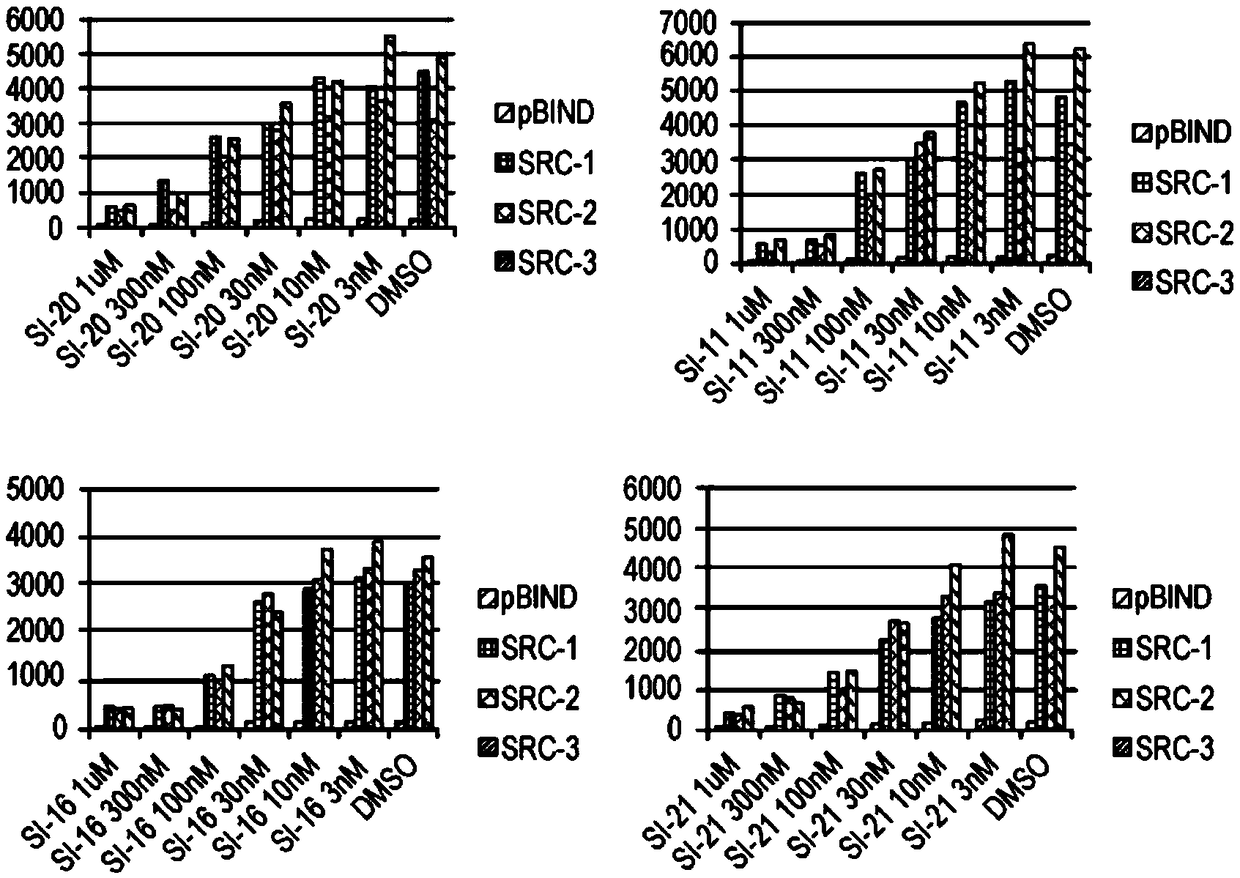
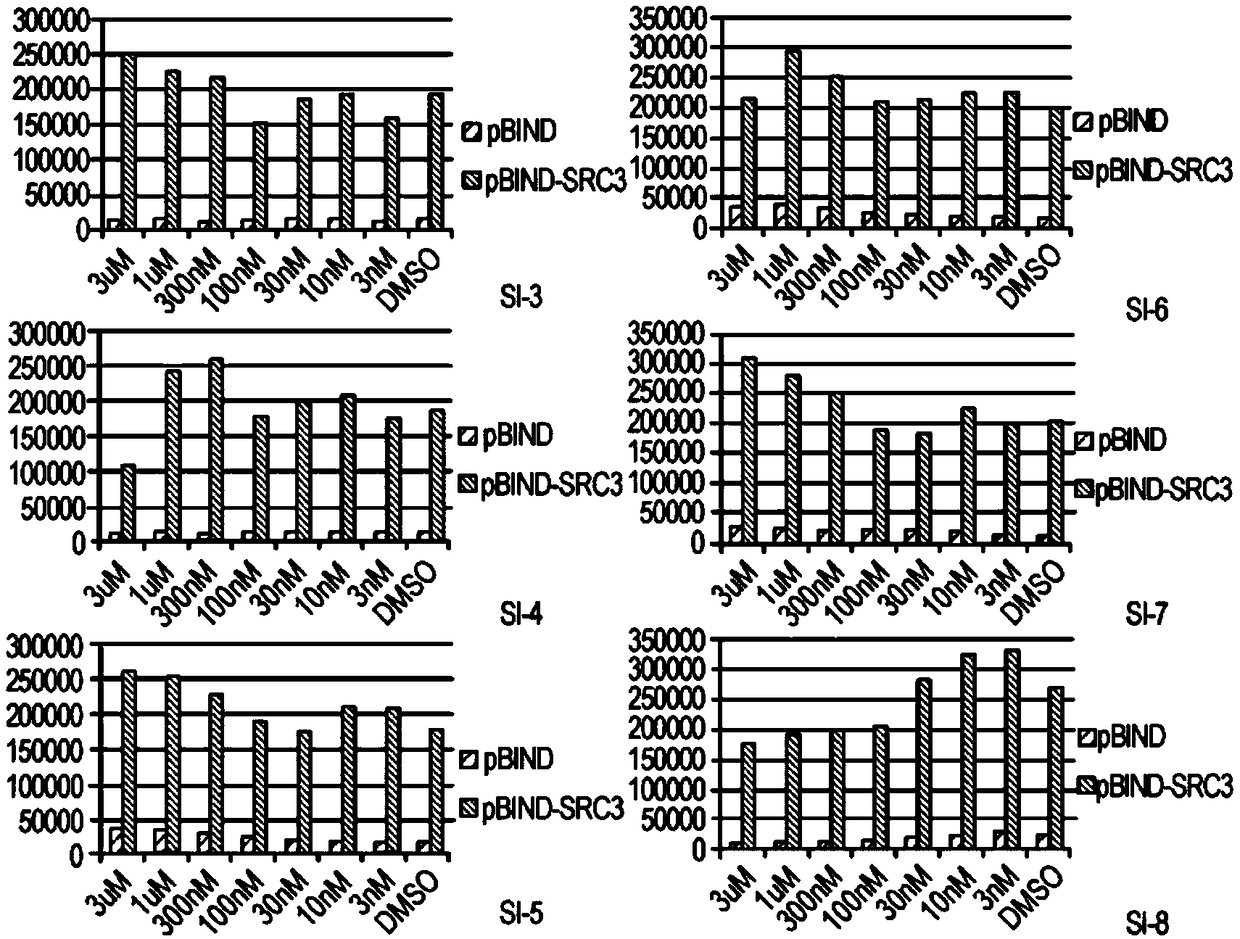
[0431] SI compounds are effective in cancer cell lines
[0432] The compounds described herein were synthesized as described in Example 1. ICs were measured in MDA-MB-468, BT-474 and MCF-7 cells by incubating a range of concentrations of the corresponding compounds for 72 hours 50 value. The results are shown in Table 1.
[0433] Table 1
[0434] compound
MDA-MB-468
BT-474
MCF-7
SI-3
NA*
SI-4
NA*
SI-5
NA*
SI-6
++
SI-7
NA*
SI-8
NA*
SI-9
+++
SI-10
+++
+++
SI-11
+++
+++
...
Embodiment 3
[0443] SI-12 was tested in pancreatic, lung, brain and melanoma cancers. Representative examples of pancreatic tumor treatment are described below. Panc-1 cells stably expressing firefly luciferase (Panc-1-Luc, 0.5×10 6 cells / mouse) into the tail of the pancreas of SCID mice (6-7 weeks). Mice were randomly divided into two groups (n=8 / group) based on luciferase imaging. Eight days after tumor inoculation, both groups were administered SI-12 (10 mg / kg, ip injection, once daily) or an equal volume of vehicle control five times a week. Tumor growth and systemic toxicity were monitored based on luciferase imaging and weekly body weight. Mice were sacrificed after 8 weeks of treatment. SI-12 treatment significantly delayed pancreatic tumor growth ( image 3 A and 3C), while causing minimal weight loss ( image 3 B). At the end of the experiment, tumors were harvested and weighed. The average tumor weight in SI-12-treated mice was approximately 30% that of the vehicle-treate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com