Catalyst used for synthesizing cyclic dipeptide in one step and application
A catalyst and cyclic dipeptide technology, applied in the fields of fine organic synthesis and biopharmaceutical synthesis, can solve the problems of difficult control of three wastes, complicated post-processing process, low final yield, etc. The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
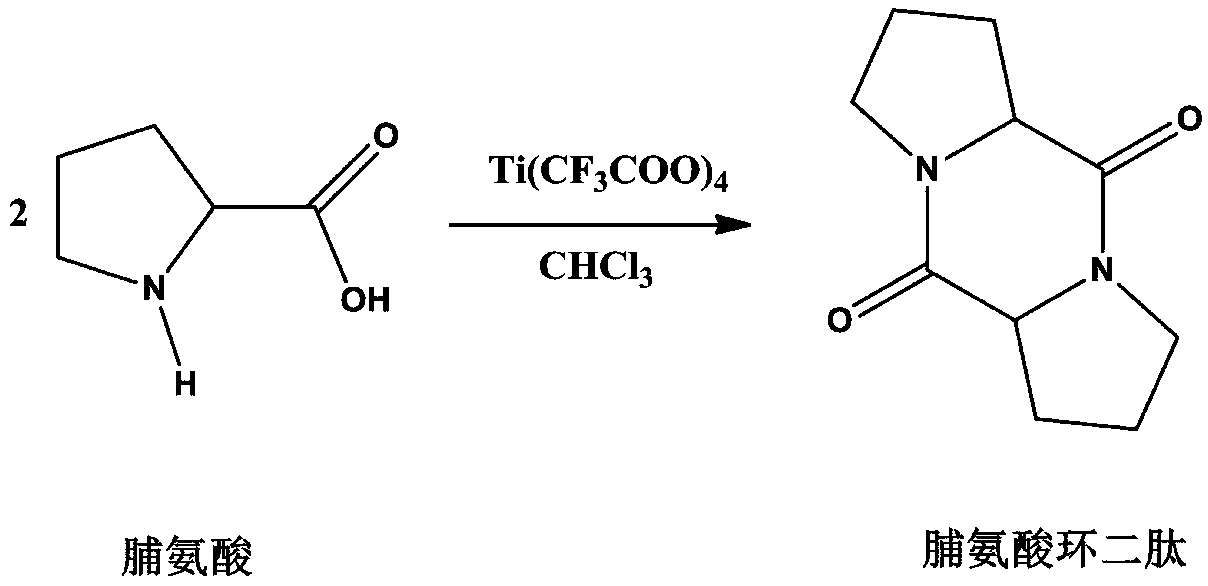
Embodiment 4
[0038] Embodiment one titanium trichloride is prepared as the catalyst of metal titanium compound (catalyst A)
[0039] Add 150ml of titanium trichloride aqueous solution with a content of 15-20% into a 500ml four-neck bottle (stirring, dropping funnel, hydrogen chloride airway tube), and add dropwise a mixture of 50g of trifluoromethanesulfonic acid and 50ml of deionized water under stirring. The solution was dropped in about half an hour, stirred at room temperature for 24 hours, filtered to remove impurities, and the mother liquor was distilled under reduced pressure at 0.09~-0.1Mpa and 60~80°C until no liquid slipped out, and the residue was dried in an evaporating dish at 120°C After 10 hours, about 60 g of Catalyst A was obtained after pulverization, which was sealed and dried for storage.
Embodiment 6
[0040] Embodiment two titanium tetrachlorides prepare as the catalyst of metal titanium compound (catalyst B)
[0041] Add 114g (1mol) of trifluoromethylacetic acid to a 250ml three-necked flask (hydrogen chloride airway, stirring, dropping funnel), and slowly drop in 30ml (about 50g, 0.25mol) of titanium tetrachloride while stirring, and drop it in about 1 hour , the system gradually solidified during the process. After 2 hours, the hydrogen chloride was removed under reduced pressure, and dried at 110-130° C. for 6 hours to obtain about 120 g of catalyst B, which was sealed and dried.
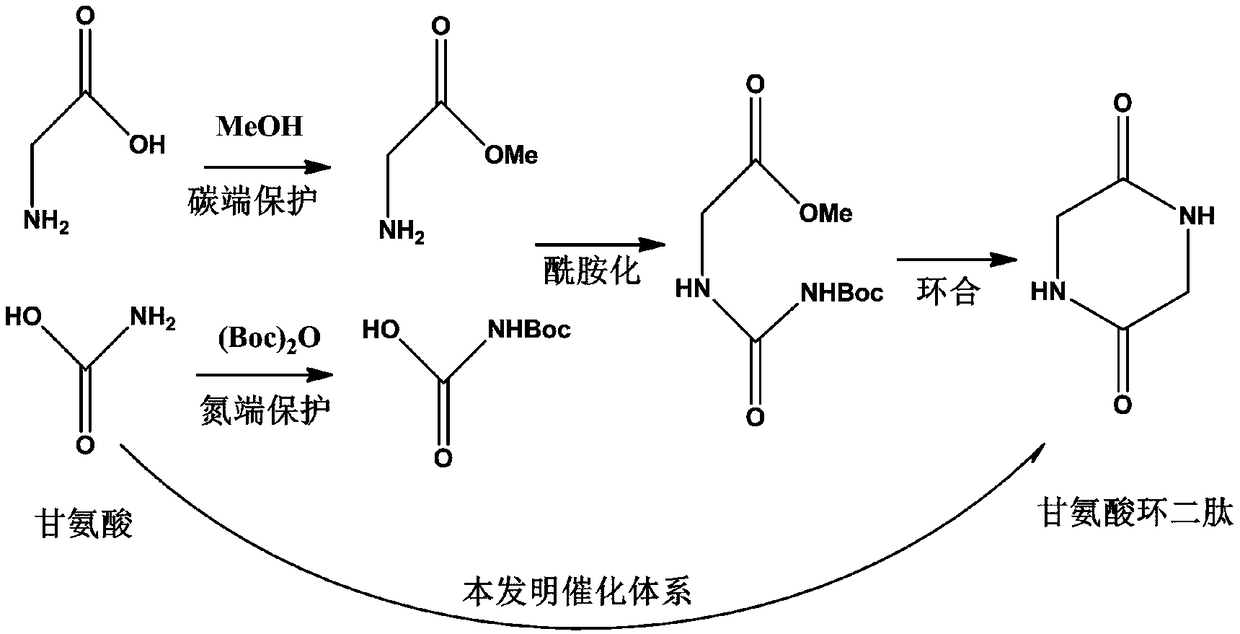
[0042] 2. One-step catalytic synthesis of cyclic dipeptides from amino acids
[0043] Utilizing the catalytic system prepared by the present invention, compared with the conventional synthesis method, it can catalyze amino acids to synthesize cyclic dipeptides in one step without the protection of carbon or nitrogen ends: now take the synthesis of symmetrical cyclic dipeptides from glycine as...
Embodiment 3
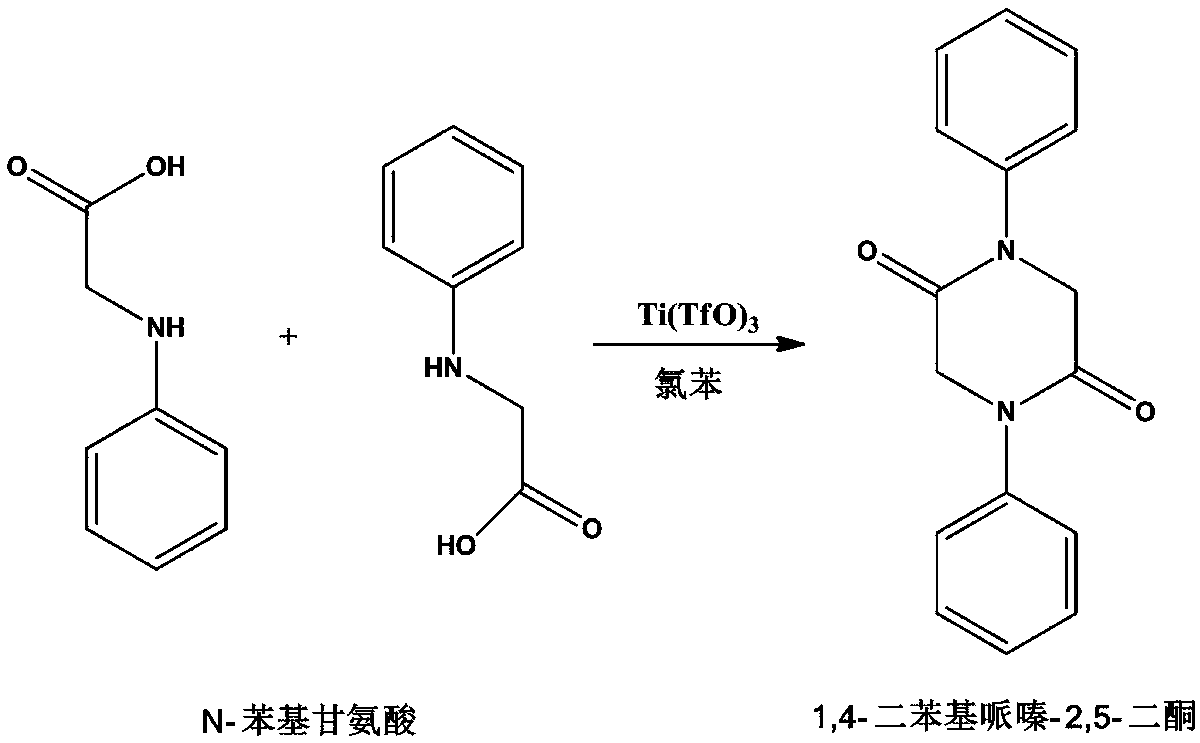
[0046] Example Triphenylglycine cyclization to prepare 1,4-diphenylpiperazine-2,5-dione
[0047] like figure 2 Shown is a synthetic route diagram of 1,4-diphenylpiperazine-2,5-dione prepared by cyclization of phenylglycine according to a specific embodiment of the present invention.
[0048] In a 500ml four-neck flask (stirring, thermometer, reflux condenser, feeding port), add 200ml of chlorobenzene, add 30g (0.2mol) of N-phenylglycine under stirring, raise the temperature to 130°C, and after the solid is completely dissolved, add 0.15 g catalyst B, kept reflux for 8 hours, cooled, filtered, the filter cake was washed with a small amount of chlorobenzene, and the mother liquors were combined and recovered. The filter cake was vacuum-dried at 80°C for 1 hour, cooled, and the dried filter cake was fully washed with 50ml of deionized water, filtered, and the mother liquor was recovered and reused. After repeated use for 6 times, the water was removed and the catalyst was recov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com