Functionalized ionic liquid ligand used for CuAAC reaction, and preparation method and applications thereof
An ionic liquid and functionalization technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problems that functionalized ionic liquid ligands have not been reported, and achieve high efficiency The effect of promoting CuAAC reaction in water phase, good water solubility and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 25mL round-bottomed flask, add 2mmol of tripropargylamine, 8mmol of 1-azido-3-chloropropane, 0.4mmol of cuprous iodide, 10mL of acetonitrile and 4mmol of DIPEA, seal it with a rubber stopper, and stir at room temperature for 24 hours , filtered after the reaction was completed, the solvent and DIPEA were removed by rotary evaporation under reduced pressure, and the pure chloro-click product was obtained by silica gel column chromatography, and the separation yield was 86%.
Embodiment 2
[0023] In a 25mL round-bottomed flask, add 1mmol of the chloro-click product, 6mmol of sodium azide and 10mL of DMF successively, filter after the reaction, remove the DMF by rotary evaporation under reduced pressure, and obtain the pure azide-based click product by silica gel column chromatography. Yield 95%.
Embodiment 3
[0025]
[0026]
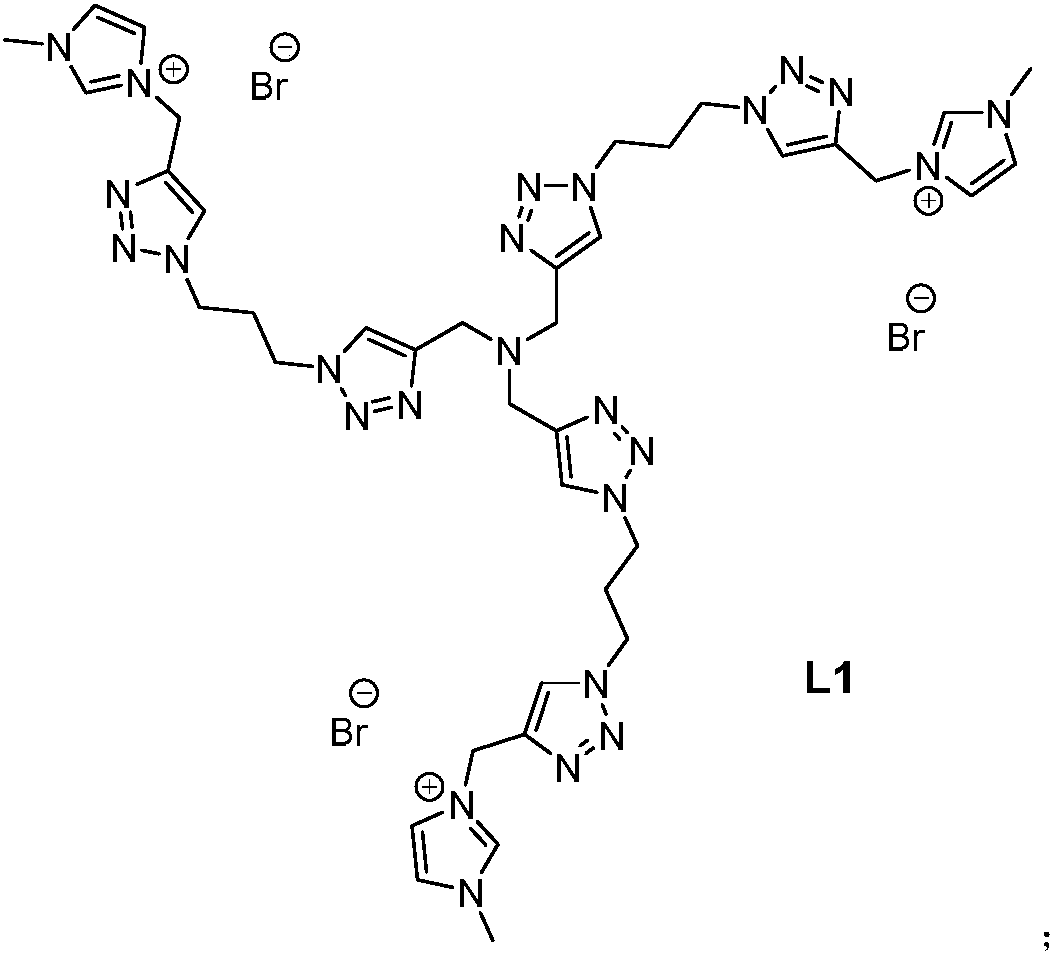
[0027] Add 0.5mmol of azide-based click product, 1.5mmol of imidazolynyl functionalized ionic liquid, 0.2mmol of cuprous iodide, 5mL of acetonitrile and 2mmol of DIPEA in a 25mL round-bottomed flask, seal with a rubber stopper, and stir at 60°C for 24 Hours, after the reaction was completed, it was filtered, and the solvent and DIPEA were removed by rotary evaporation under reduced pressure, extracted with ether and water, the water phase was filtered again, rotary evaporated under reduced pressure, and then vacuum-dried at 60°C to obtain the functionalized imidazole ionic liquid ligand L1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com