Small molecule prodrugs bridged by redox double-sensitive bonds and their self-assembled nanoparticles
A technology of self-assembling nanoparticles and small molecules, which is applied in the direction of drug combination, effective ingredients of hydroxyl compounds, drug delivery, etc., to achieve high-efficiency entrapment, simple synthesis method, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
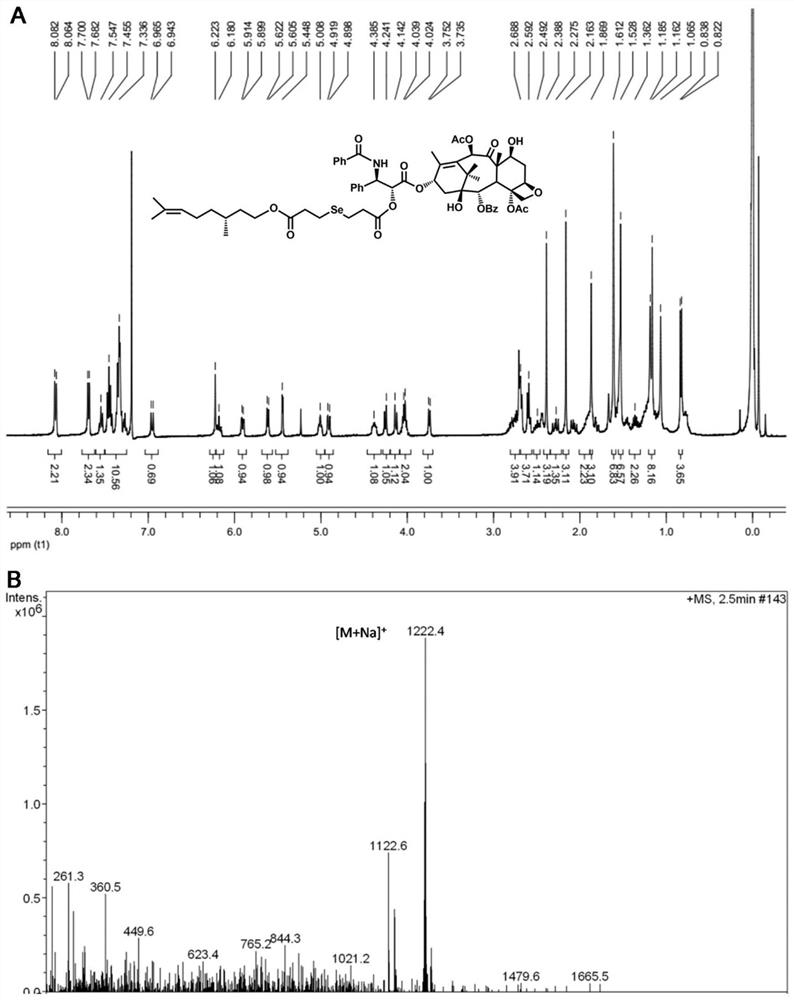
[0053] Embodiment 1: the synthesis of monoselenium bridged paclitaxel-citronellol small molecule prodrug (PTX-Se-CIT)
[0054] Add an appropriate amount of selenodipropionic acid into a 50mL round-bottomed flask, and dissolve it with 3mL of acetic anhydride, stir at room temperature for 2 hours, monitor the reaction process by thin-layer chromatography, and then add 20mL of toluene to the system three times, and reduce Distilled to dryness. The obtained product was dissolved in 30 mL of dichloromethane, and an appropriate amount of citronellol and DMAP was added, stirred at room temperature for 1 hour, the reaction process was monitored by thin-layer chromatography, and an intermediate product was obtained by purification by silica gel column chromatography. Finally, the intermediate product, EDCI, HOBt and DMAP were dissolved in 50 mL of anhydrous dichloromethane, ice-bathed for 1 hour, then an appropriate amount of paclitaxel was added, stirred at room temperature for anothe...
Embodiment 2
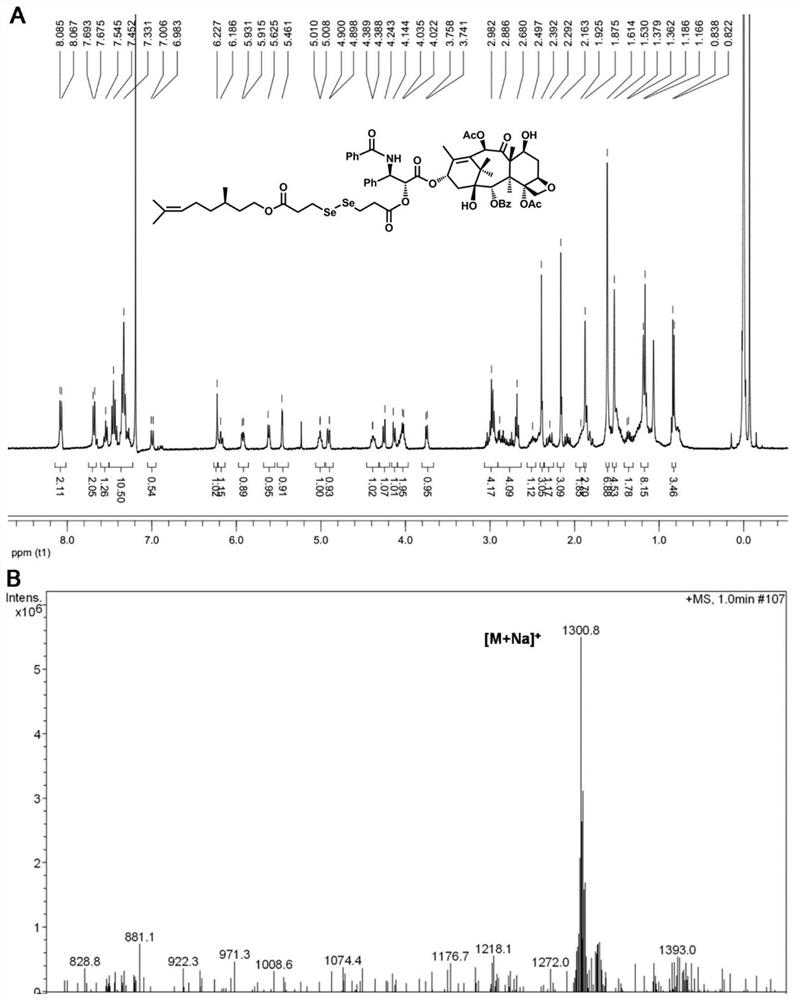
[0057] Example 2: Synthesis of diselenide bridged paclitaxel-citronellol small molecule prodrug (PTX-SeSe-CIT)
[0058] Add an appropriate amount of 3,3'-diselenodipropionic acid into a 50mL round-bottomed flask, dissolve it with 3mL of acetic anhydride, stir at room temperature for 2 hours, monitor the reaction process by thin-layer chromatography, and then add 20mL of toluene three times system, and evaporated to dryness under reduced pressure. The obtained product was dissolved in 30 mL of dichloromethane, and an appropriate amount of citronellol and DMAP was added, stirred at room temperature for 1 hour, the reaction process was monitored by thin-layer chromatography, and an intermediate product was obtained by purification by silica gel column chromatography. Finally, the intermediate product, EDCI, HOBt and DMAP were dissolved in 50 mL of anhydrous dichloromethane, ice-bathed for 1 hour, then an appropriate amount of paclitaxel was added, stirred at room temperature for ...
Embodiment 3
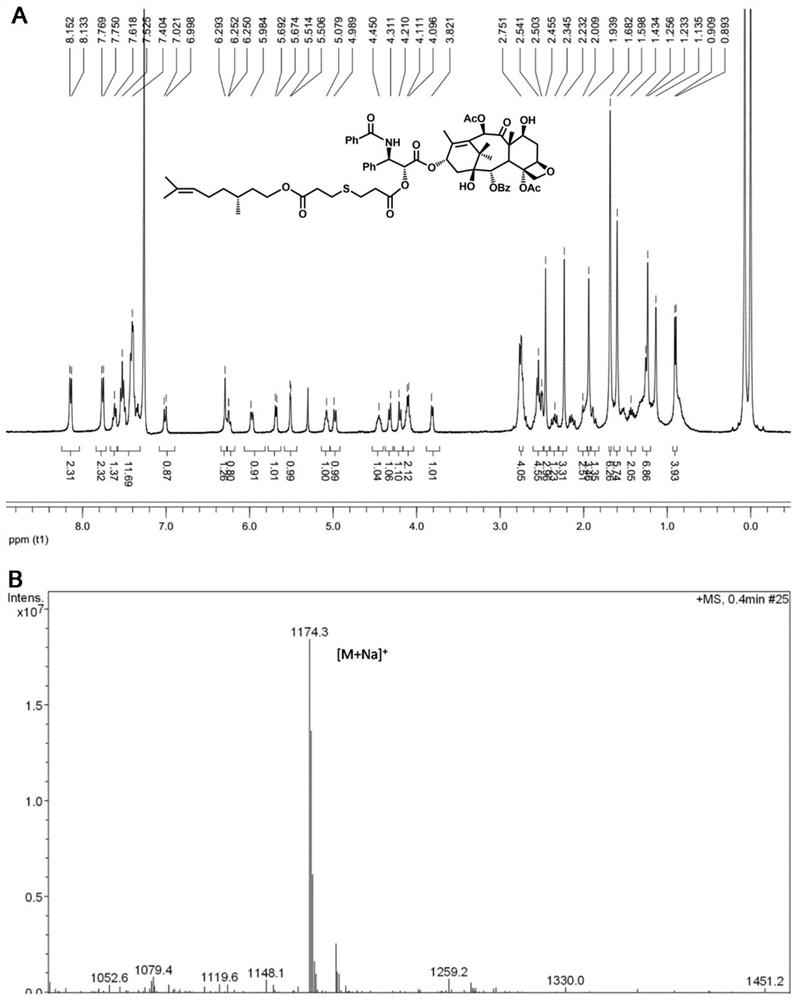
[0061] Embodiment 3: Synthesis of monosulfide bridged paclitaxel-citronellol small molecule prodrug (PTX-S-CIT)
[0062] Add an appropriate amount of thiodipropionic acid into a 50mL round-bottomed flask, dissolve it with 3mL of acetic anhydride, stir at room temperature for 2 hours, monitor the reaction process by thin-layer chromatography, then add 20mL of toluene to the system three times, and reduce Distilled to dryness. The obtained product was dissolved in 30 mL of dichloromethane, and an appropriate amount of citronellol and DMAP were added, stirred at room temperature for 1 hour, the reaction process was monitored by thin-layer chromatography, and an intermediate product was obtained by purification by silica gel column chromatography. Finally, the intermediate product, EDCI, HOBt and DMAP were dissolved in 50 mL of anhydrous dichloromethane, ice-bathed for 1 hour, then an appropriate amount of paclitaxel was added, stirred at room temperature for another 24 hours, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com