Polysubstituted 2-aminopyridine compound and application thereof in preparation of anti-tumor drugs
An anti-tumor drug, aminopyridine technology, applied in the field of pharmacy, can solve the problems that specific WSB-1 inhibitors have not yet been reported, and achieve significant anti-tumor metastasis effect, high specificity, and novel mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation method and structural characterization of polysubstituted 2-aminopyridine compounds
[0032] The preparation of polysubstituted 2-aminopyridine compounds refers to patents (WO2005100353, WO2016135048) and literature (Lingam VS, Dahale DH, Rathi VE et al. Design, Synthesis, and Pharmacological Evaluation of 5,6-Disubstituted Pyridin-2(1H) -one Derivativesas Phosphodiesterase 10A(PDE10A) Antagonists.J Med Chem.2015,58(20):8292-8308.) The synthesis method, through simple Miyaura boronation reaction, Suzuki coupling reaction, reductive amination, condensation reaction And so on. The structure characterization data of the prepared polysubstituted 2-aminopyridine compounds are as follows:
[0033] 5,6-Bis(3-methyl4-methoxyphenyl)-2-aminopyridine (I-1)
[0034] 1 H NMR(500MHz, CDCl 3 )δ7.44(d,J=8.3Hz,1H), 7.25(d,J=1.5Hz,1H), 7.00(dd,J=8.4,2.1Hz,1H), 6.94(d,J=1.6Hz, 1H), 6.85 (dd, J = 8.4, 2.1 Hz, 1H), 6.66 (d, J = 8.4 Hz, 1H), 6.61 (d, J = 8.5 Hz, 1H), 6.49 ...
Embodiment 2
[0043] Example 2: Evaluation of the inhibitory activity of polysubstituted 2-aminopyridine compounds on tumor cell migration in vitro
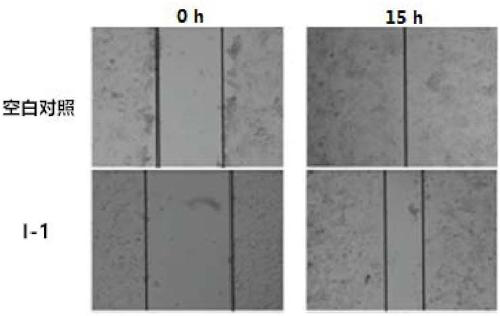
[0044] The present invention uses models based on phenotypic screening, namely the scratch experiment and the Transwell chamber experiment, to determine the ability of polysubstituted 2-aminopyridine compounds to inhibit tumor cells from repairing scratches and to inhibit tumor cells from migrating through the Transwell membrane, thereby To evaluate the ability of the test compound to inhibit tumor cell migration in vitro. The cell strain used in the present invention is human osteosarcoma cell KHOS, and the experimental results are based on cell coverage and cell migration as parameters.
[0045] Scratch test method: In a hypoxic environment, inoculate osteosarcoma cells KHOS in a 24-well culture plate, and when they are fully confluent (dense monolayer cells), use a 10μl tip to scratch each well of the monolayer cells Create a wound model of cul...
Embodiment 3
[0056] Example 3: Compound I-1 for WSB-1-RhoGDI 2 Pathway inhibitory activity measurement
[0057] In the present invention, the inhibitory activity of compound I-1 on WSB-1-RhoGDI2 signal pathway was investigated by Western Blot method.
[0058] Experimental method: Inoculate H1299 cells stably transformed with WSB-1 in a 24-well culture plate, incubate with test compound I-1 (5μM) for 48 hours under hypoxia, collect the cells, and use DMSO as a blank control group , The Western Blot method was used to analyze the degree of inhibition of WSB-1 and RhoGDI2 protein ubiquitination degradation in the administration group.
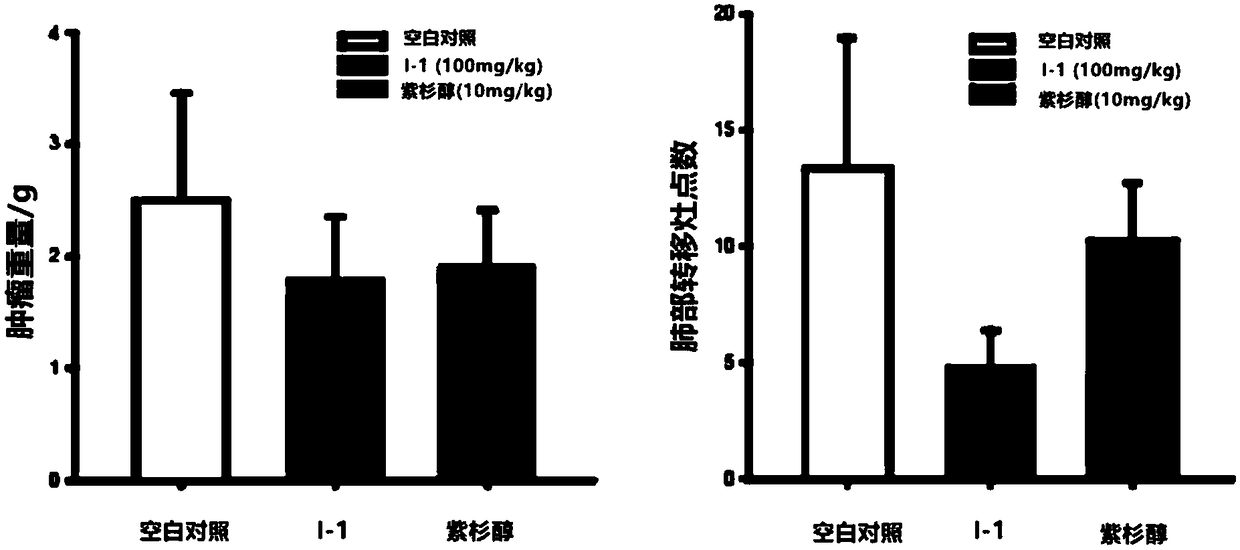
[0059] The experimental results are as figure 2 Shown. Compound I-1 can significantly inhibit the expression of WSB-1 at a concentration of 5 μM (the inhibition rate is about 72%), thus increasing the expression level of the downstream protein RhoGDI2, while no obvious cytotoxicity (normal cell morphology) is observed. The experimental results show that compound I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com