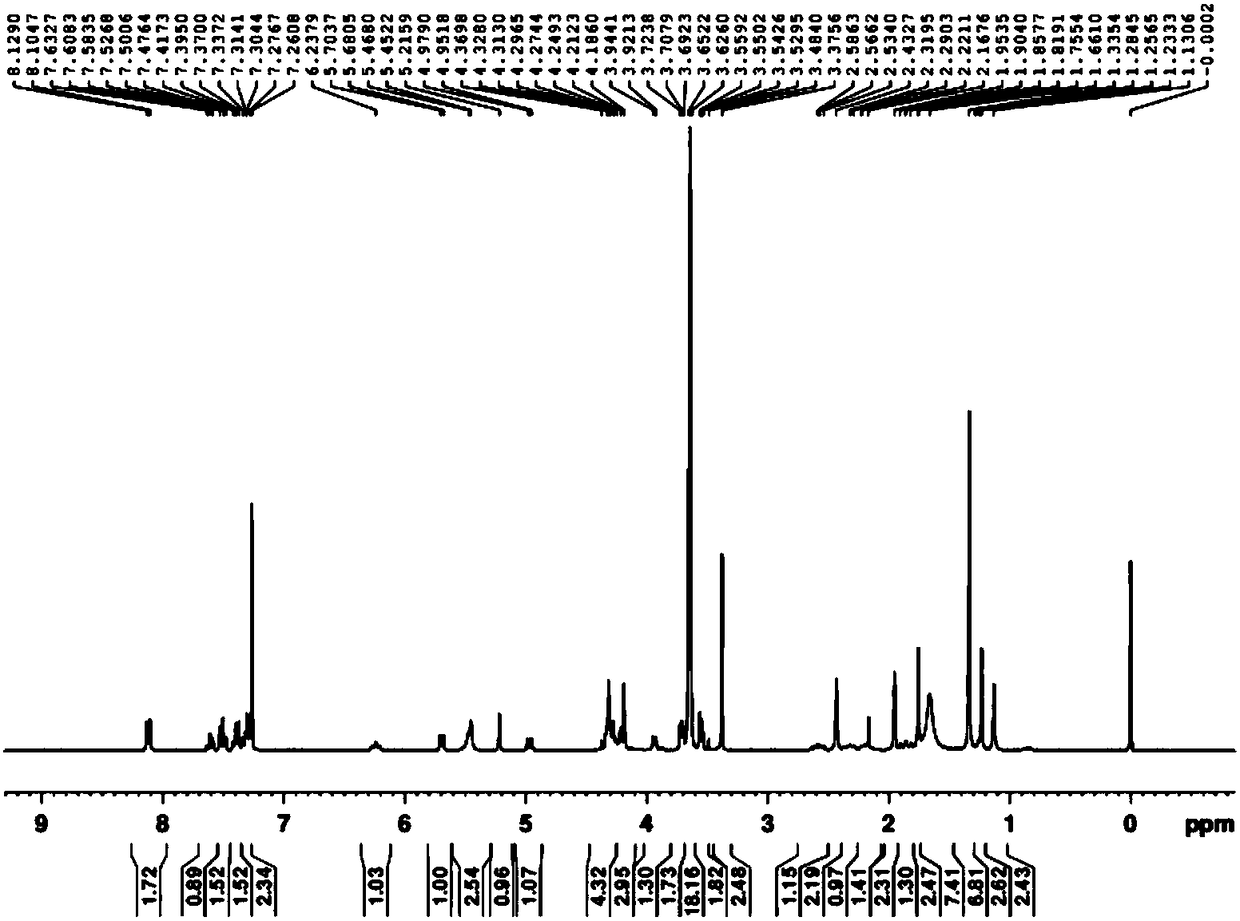
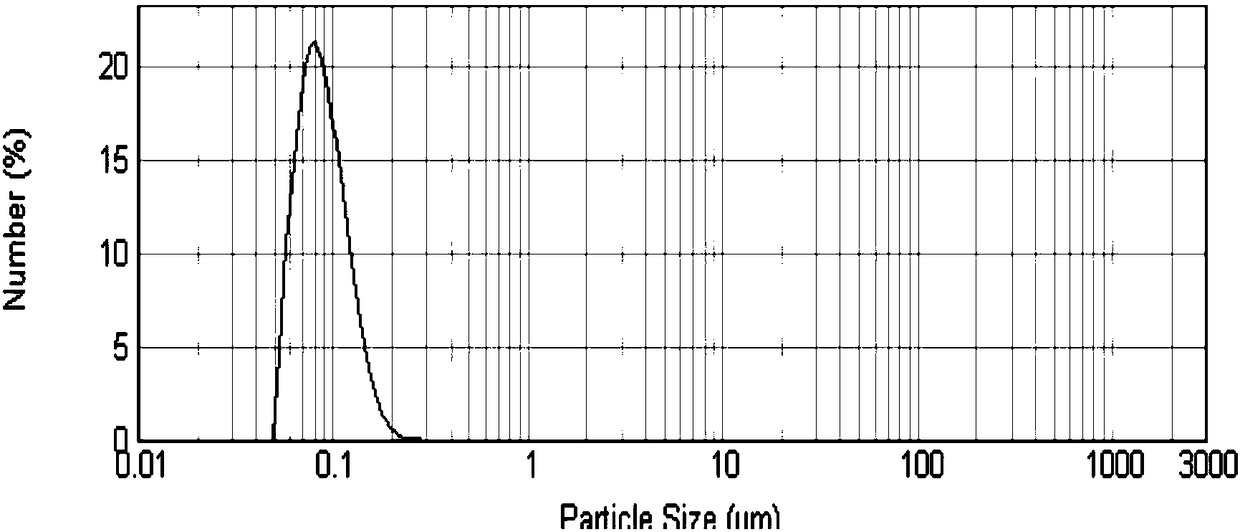
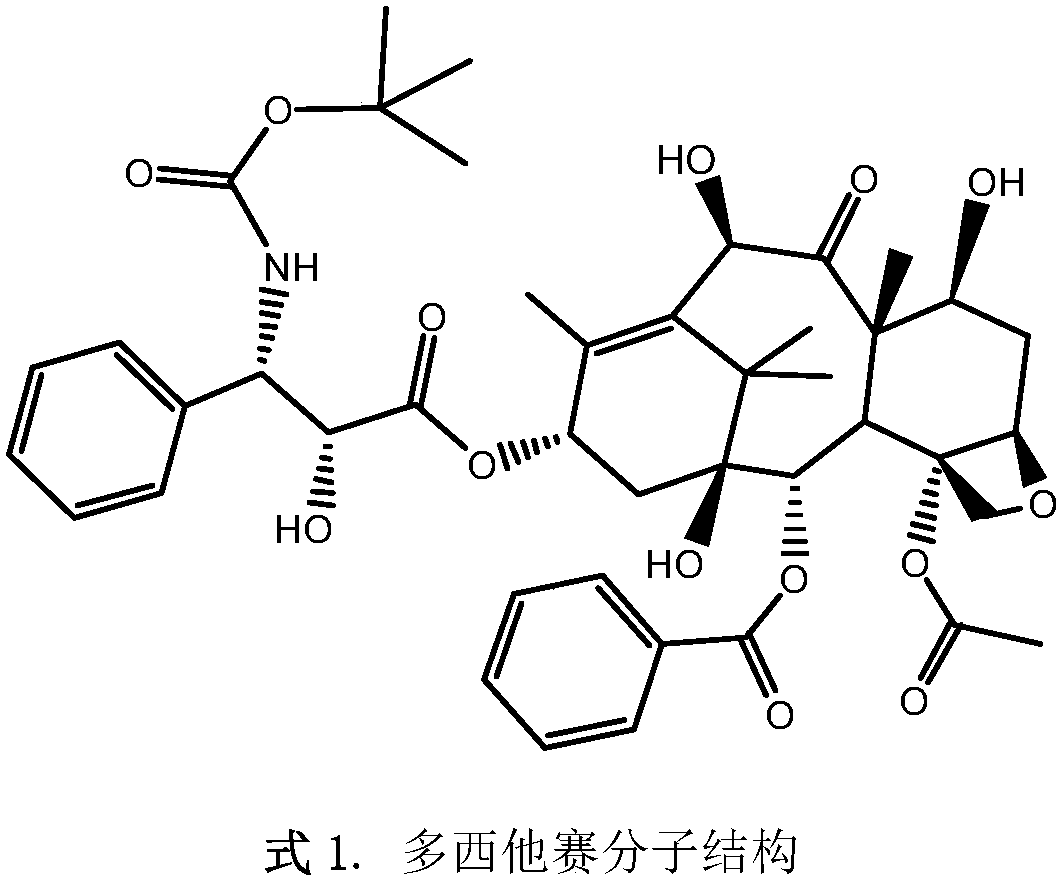
Injection of oligoethylene glycol-modified docetaxel derivative
A technology of oligoethylene glycol and docetaxel, which is applied in the field of injection of docetaxel derivatives, and can solve the problems of low drug loading, high toxicity, and poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 8
[0081] Embodiment 1. the synthesis of octapolyethylene glycol monomethyl ether docetaxel-2'-diglycolate
[0082] The synthesis of the docetaxel derivative comprises the following steps:
[0083] 1) Synthesis of octapolyethylene glycol monomethyl ether diglycolate
[0084] The reaction formula is as follows:
[0085]
[0086] Experimental steps:
[0087] Method 1: In a 100mL round bottom flask, add 2.306g (6mmol) of dried octapolyethylene glycol monomethyl ether (1), 1.044g (9mmol) of diglycolic anhydride and 200mg of tin 2-ethylhexanoate, Add 30 mL of toluene, stir, and heat to reflux under nitrogen protection until the reaction is complete. After removing the toluene in the reaction solution with a rotary evaporator, add 20 mL of ethyl acetate, stir, and then add 10 mL of diethyl ether, a white solid precipitates, remove the solid matter by filtration, concentrate the filtrate to 10 mL with a rotary evaporator, and separate by column layer to obtain 2. 306g octapolyeth...
Embodiment 2
[0094] Embodiment 2. Synthesis of hexaethylene glycol monomethyl ether docetaxel-2'-diglycolate
[0095] The synthesis of the docetaxel derivative comprises the following steps:
[0096] 1) Synthesis of Hexaethylene Glycol Monomethyl Ether Diglycolate
[0097] The reaction formula is as follows:
[0098]
[0099] Experimental steps:
[0100] In a 100mL round bottom flask, add 1.7787g (6mmol) of dried hexapolyethylene glycol monomethyl ether (5), 1.044g (9mmol) of diglycolic anhydride and 200mg of tin 2-ethylhexanoate, and then add 30mL toluene, stirred, and heated to reflux under nitrogen protection until the reaction was complete. After removing the toluene in the reaction solution with a rotary evaporator, add 20 mL of ethyl acetate, stir, and then add 10 mL of diethyl ether, a white solid precipitates, remove the solid matter by filtration, concentrate the filtrate to 10 mL with a rotary evaporator, and separate by column layer to obtain 1.914g hexapolyethylene glyco...
Embodiment 3
[0106] Example 3. Synthesis of tetrapolyethylene glycol monomethyl ether docetaxel-2'-diglycolate
[0107] The synthesis of the docetaxel derivative comprises the following steps:
[0108] 1) Synthesis of tetrapolyethylene glycol monomethyl ether diglycolate
[0109] The reaction formula is as follows:
[0110]
[0111] Experimental steps:
[0112] In a 100mL round bottom flask, add 1.250g (6mmol) of dried tetrapolyethylene glycol monomethyl ether (9), 1.044g (9mmol) of diglycolic anhydride and 200mg of tin 2-ethylhexanoate, and then add 30mL toluene, stirred, and heated to reflux under nitrogen protection until the reaction was complete. After removing the toluene in the reaction solution with a rotary evaporator, add 20 mL of ethyl acetate, stir, and then add 10 mL of diethyl ether, a white solid precipitates, remove the solid matter by filtration, concentrate the filtrate to 10 mL with a rotary evaporator, and separate by column layer to obtain 1.650g tetrapolyethyle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com