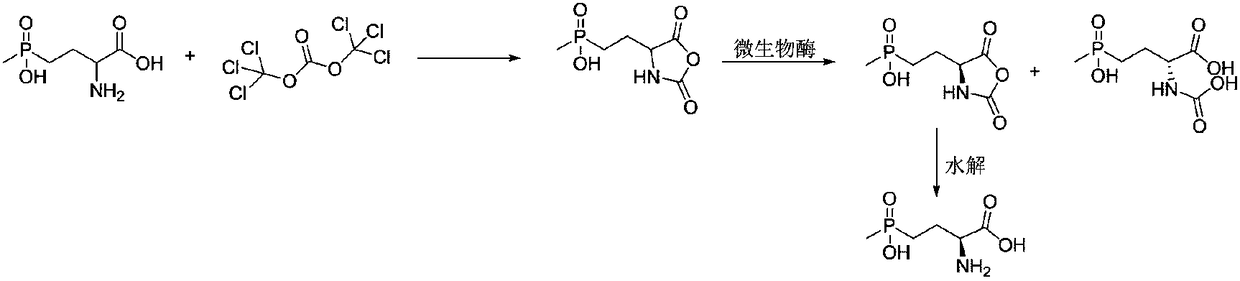
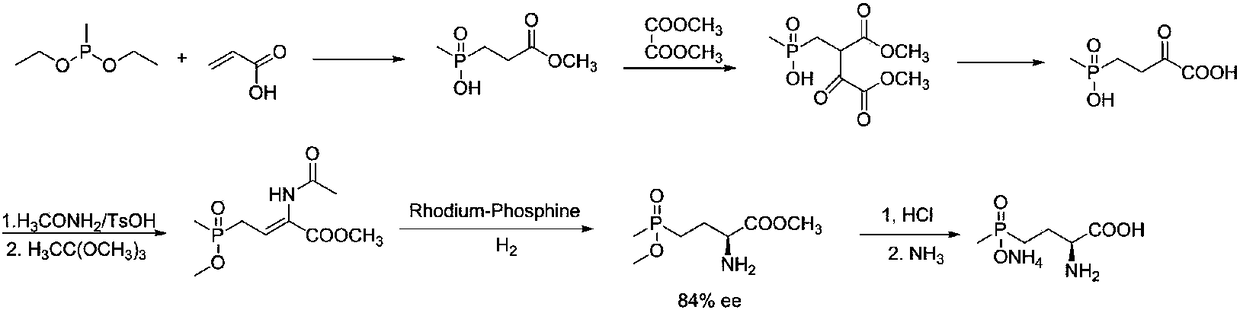
L-glufosinate ammonium preparation method
A glufosinate-ammonium and phosphonic acid-based technology, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, and bulk chemical production, can solve the problems of low total yield, high price, and difficulty in separation and purification and other issues, to achieve the effect of easy industrialization, industrialization and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: (R=Et)
[0048] Take 760mg (4mmol) of the glufosinate-ammonium intermediate and dissolve it in 40ml of PBS buffer (0.2M, pH=8.0) solution. Add 20 mg of pyridoxal phosphate and 50 mg of nitrile hydratase (purchased from Suzhou Pilot Biotechnology Co., Ltd., product number YH1603) dissolved in 1 ml of water in turn to the reaction solution. Only one model of the product is given here for illustration. Effect of the invention), 38 mg of L-amide hydrolase dissolved in 1 ml of water (purchased from Suzhou Pilot Biotechnology Co., Ltd., product number is YH1411, only one of the models is given here to illustrate the effect of the present invention), 76 mg of ACL racemase dissolved in 1 ml of water (purchased from Suzhou Pilot Biotechnology Co., Ltd., product number is YH1507, only one model of the product is given here to illustrate the effect of the present invention). After the addition, the temperature of the system was raised to 30° C., and the reaction was...
Embodiment 2
[0049] Embodiment 2: (R=n-Bu)
[0050] Dissolve 872mg (4mmol) of glufosinate-ammonium intermediate in 40ml of PBS buffer (0.2M, pH=8.0) solution. Add 20 mg of pyridoxal phosphate and 50 mg of nitrile hydratase (purchased from Suzhou Pilot Biotechnology Co., Ltd., product number YH1603) dissolved in 1 ml of water in turn to the reaction solution. Only one model of the product is given here for illustration. Effect of the invention), 38 mg of L-amide hydrolase dissolved in 1 ml of water (purchased from Suzhou Pilot Biotechnology Co., Ltd., product number is YH1411, only one of the models is given here to illustrate the effect of the present invention), 87 mg of ACL racemase dissolved in 1 ml of water (purchased from Suzhou Pilot Biotechnology Co., Ltd., product number is YH1507, only one type of product is given here to illustrate the effect of the present invention). After the addition was completed, the temperature of the system was raised to 30° C., and the reaction was comp...
Embodiment 3
[0051] Embodiment 3: (R=H)
[0052]Take 648mg (4mmol) of the glufosinate-ammonium intermediate and dissolve it in 40ml of PBS buffer (0.2M, pH=8.0) solution. Add 20 mg of pyridoxal phosphate and 50 mg of nitrile hydratase (purchased from Suzhou Pilot Biotechnology Co., Ltd., product number YH1603) dissolved in 1 ml of water in turn to the reaction solution. Only one model of the product is given here for illustration. Effect of the invention), 38 mg of L-amide hydrolase dissolved in 1 ml of water (purchased from Suzhou Pilot Biotechnology Co., Ltd., product number is YH1411, only one of the models is given here to illustrate the effect of the present invention), 65 mg of ACL racemase dissolved in 1 ml of water (purchased from Suzhou Pilot Biotechnology Co., Ltd., product number is YH1507, only one model of the product is given here to illustrate the effect of the present invention). After the addition was completed, the temperature of the system was raised to 30° C., and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com