Two Penicillin Glucosides and Their Application in Anti-Kidney Cancer Drugs
A technology of tetromycin and glucoside, applied in the field of microbial natural products, can solve the problems of weak mechanism of action, and the anti-tumor potential needs to be further explored. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of Penicillin glucoside glucopiericidin A and 7-demethylglucopiericidinA
[0025] 1. Solid culture of Streptomyces sp.HBERC-58855
[0026] Streptomyces sp.HBERC-58855 (preservation number is CCTCC NO:M 2017186) is isolated from the mangrove bottom mud, and the strain is preserved on the slant of ISP-2 medium. The composition of ISP-2 medium is: yeast extract powder 4g, glucose 4g, malt extract powder 10g, coarse sea salt 30g, agar powder 20g, water 1000mL, pH 7.2-7.4, sterilized for later use.
[0027] 2. Amplified fermentation of Streptomyces sp.HBERC-58855
[0028] Take a small amount of Streptomyces sp. HBERC-58855 strain on the slant for seed fermentation. The medium is 20 grams of mannitol, 10 grams of soybean peptone, 2.5 grams of soybean oil, 0.35 grams of dipotassium hydrogen phosphate, 950 ml of deionized water, adjusted to pH 7.0, and the volume is adjusted to 1000 ml, and 100 ml of 500 ml Erlenmeyer flask is sterilized spare. The cu...
Embodiment 2
[0031] Example 2: Identification of the structures of Glucopiericidin A and 7-demethylglucopiericidin A
[0032] 1. Identification of Glucopiericidin A structure
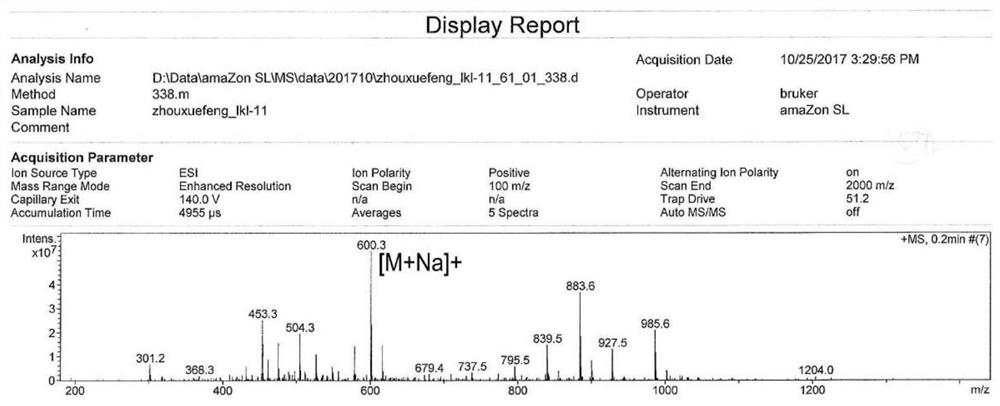
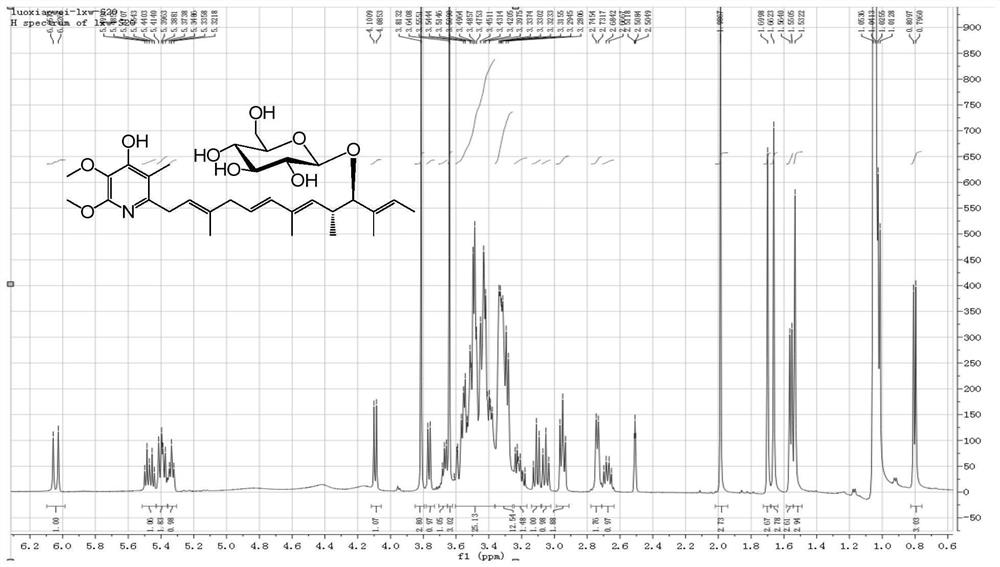
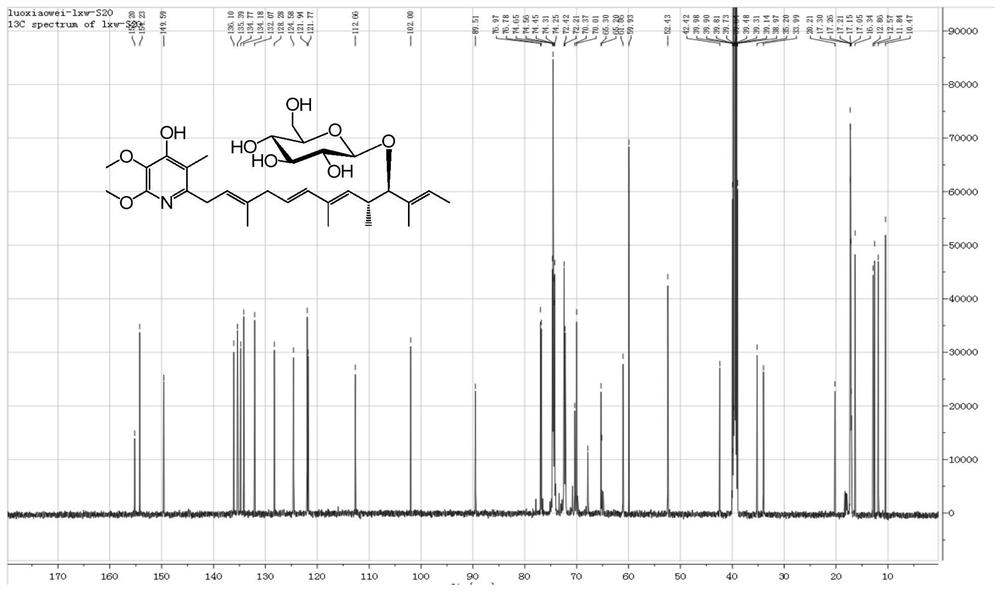
[0033] The molecular ion peak of compound 1 (Glucopiericidin A) analyzed by mass spectrometry is 600.3[M+Na] + ( figure 1 ), and its molecular weight is 577. NMR H spectrum ( figure 2 ) and C spectrum ( image 3 ) is consistent with Glucopiericidin A reported in the literature (JAntibiot.1987,40,149-156.), so the compound is identified as Glucopiericidin A.
[0034]
[0035] The glucopiericidin A of the fenopterine compound has a structural formula as shown in formula (I), wherein R=CH 3 ;
[0036] 2. Structural identification of 7-Demethylglucopiericidin A
[0037] Compound 2 (7-Demethylglucopiericidin A): Pale yellow oil, 1.95°(c 0.20, MeOH); IR(ATR)ν max 3317,2928,1472,1456,1124,1076,1016,651,592,548cm -1 ; CD (0.200mg / ml, MeOH), λmax (Δε) 236 (1.9), 200 (-3.5); 1 H and 13 C NMR data in Table 1;...
Embodiment 3
[0044] Example 3: Inhibitory activity of Glucopiericidin A and 7-demethylglucopiericidin A on renal cancer cells
[0045] Three human kidney cancer cell lines: cell lines ordered from Shanghai Cell Resource Center, Chinese Academy of Sciences: 786-O human kidney cancer cell line (Cat#TCHu186) ACHN human kidney cancer cell line (Cat#TCHu199); OS-RC-2 human kidney cancer cell line strain (Cat#TCHu40). Human renal tubular epithelial cells HK-2 were provided by the School of Pharmacy, Southern Medical University.
[0046] The same type of natural product Piericidin A (Piericidin A) and the marketed drug Sorafenib for the treatment of renal cancer were used as positive controls.
[0047] The cell inhibitory activity experiment adopts CCK-8 detection method. Collect the cells in the logarithmic growth phase, count them, resuspend the cells with complete medium, adjust the cell concentration to an appropriate concentration (determined according to the results of the cell density op...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com