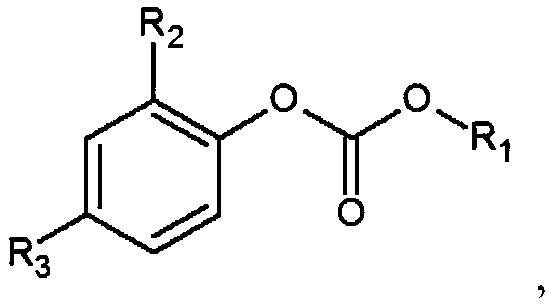
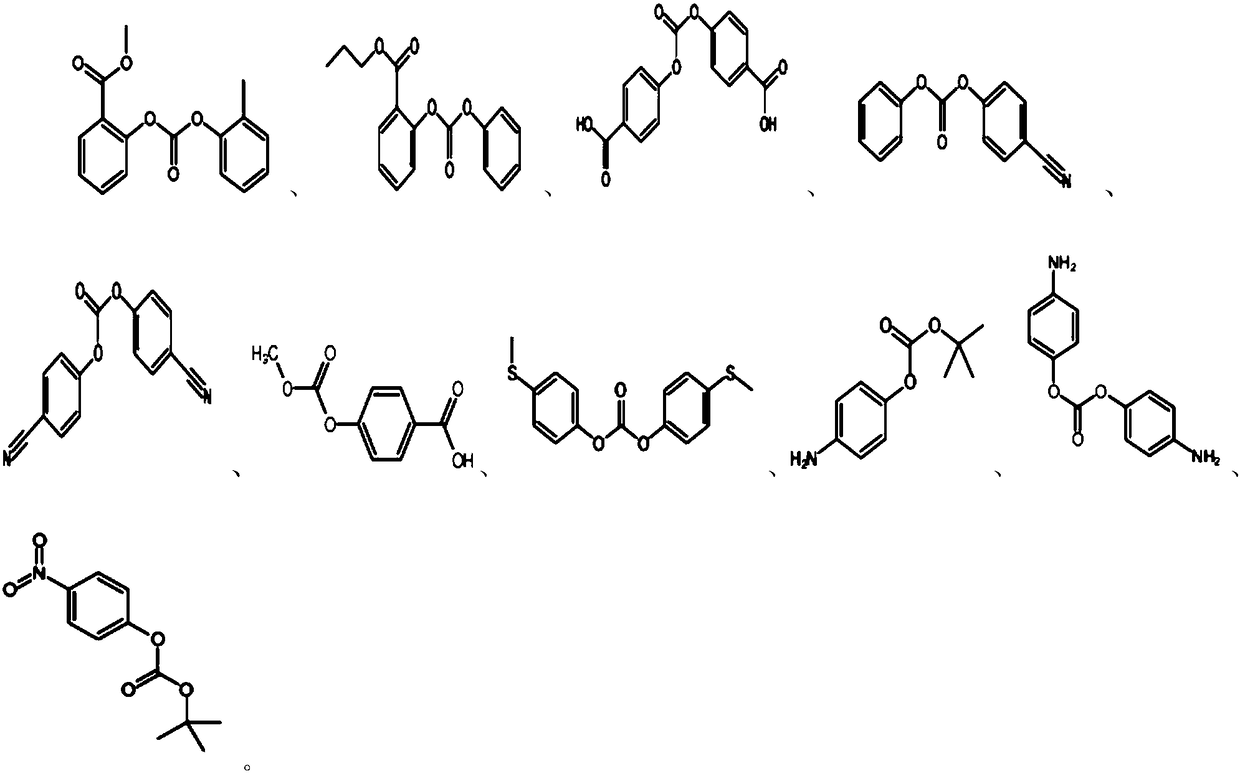
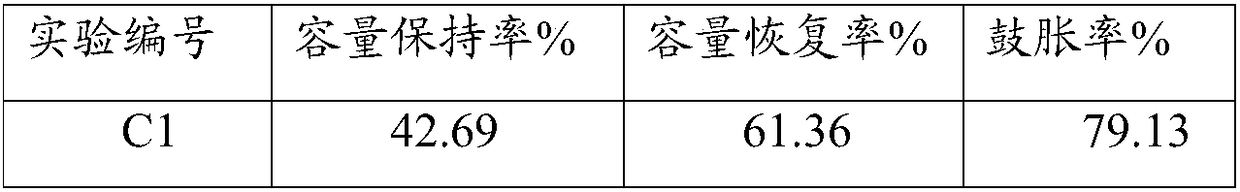
Non-aqueous electrolyte and lithium ion battery
A technology of non-aqueous electrolyte and lithium salt, which is applied in the field of electrochemistry, and can solve the problems of no examples, no specific points, no further involvement of phenyl carbonate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The organic solvents are ethylene carbonate, diethyl carbonate, ethyl methyl carbonate, and ethyl propionate, which account for 20%, 20%, 35%, and 10% of the total mass ratio of the electrolyte; the electrolyte lithium salt is LiPF 6 , and the proportion of lithium salt is 12.5%, the proportion of fluoroethylene carbonate is 0.5%, the proportion of lithium difluorooxalate borate is 0.1%, the proportion of succinic anhydride is 0.1%, and the proportion of 1,3-propane sultone is 0.2 %, the ratio of tris(trimethylsilane) phosphate is 0.1%, The proportion is 1.5%, and the electrolyte solution obtained above is applied to a 1Ah NCA polymer pouch battery, which is denoted as S1.
Embodiment 2
[0043] The organic solvents are ethylene carbonate, diethyl carbonate, ethyl methyl carbonate, and ethyl propionate, which account for 20%, 20%, 35%, and 10% of the total mass ratio of the electrolyte; the electrolyte lithium salt is LiPF 6 , and the proportion of lithium salt is 12.5%, the proportion of fluoroethylene carbonate is 0.5%, the proportion of lithium difluorooxalate borate is 0.3%, the proportion of succinic anhydride is 0.2%, and the proportion of 1,3-propane sultone is 0.3 %, the ratio of tris(trimethylsilane) phosphate is 0.2%, The ratio is 1.0%, and the electrolyte solution obtained above is applied to a 1Ah NCA polymer pouch battery, which is denoted as S2.
Embodiment 3
[0045] The organic solvents are ethylene carbonate, diethyl carbonate, ethyl methyl carbonate, and ethyl propionate, which account for 20%, 20%, 35%, and 10% of the total mass ratio of the electrolyte; the electrolyte lithium salt is LiPF 6 , and the proportion of lithium salt is 12.5%, the proportion of fluoroethylene carbonate is 0.5%, the proportion of lithium difluorooxalate borate is 0.3%, the proportion of succinic anhydride is 0.3%, and the proportion of 1,3-propane sultone is 0.3 %, the ratio of tris(trimethylsilane) phosphate is 0.3%, The ratio is 0.8%, and the electrolyte solution obtained above is applied to a 1Ah NCA polymer pouch battery, which is denoted as S3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com