N-substituted benzimidazole diamine and preparation method thereof
A technology of benzimidazole diamine and dinitro, which is applied in the field of diamine compounds and their preparation, can solve the problems of harsh conditions and high reaction temperature, and achieves the effects of easy industrialization, high reaction yield and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
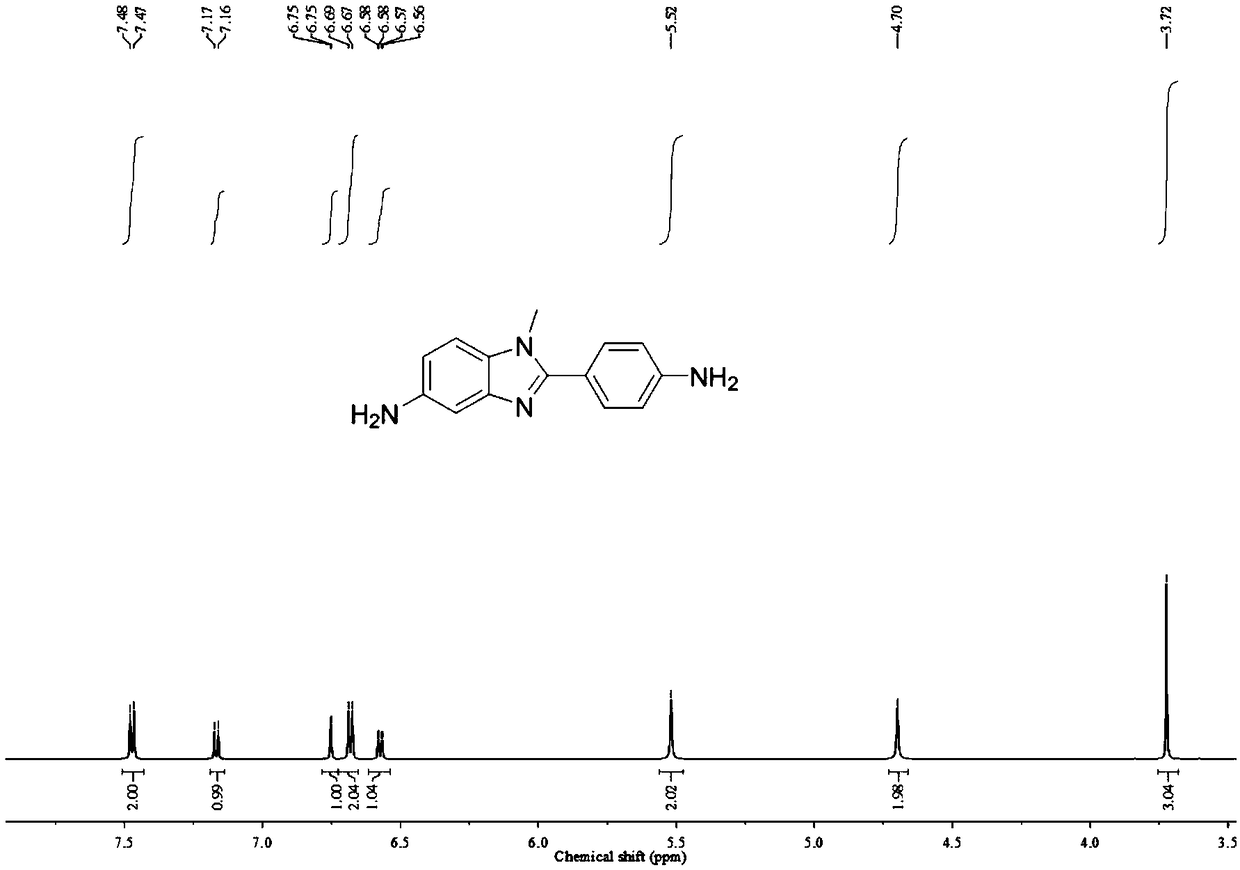
[0046] In this example, the specific structure of N-substituted benzimidazole diamine is as follows:
[0047]
[0048] The preparation method of above-mentioned 5-amino-2-(4-aminobenzene)-1-methylbenzimidazole is as follows:
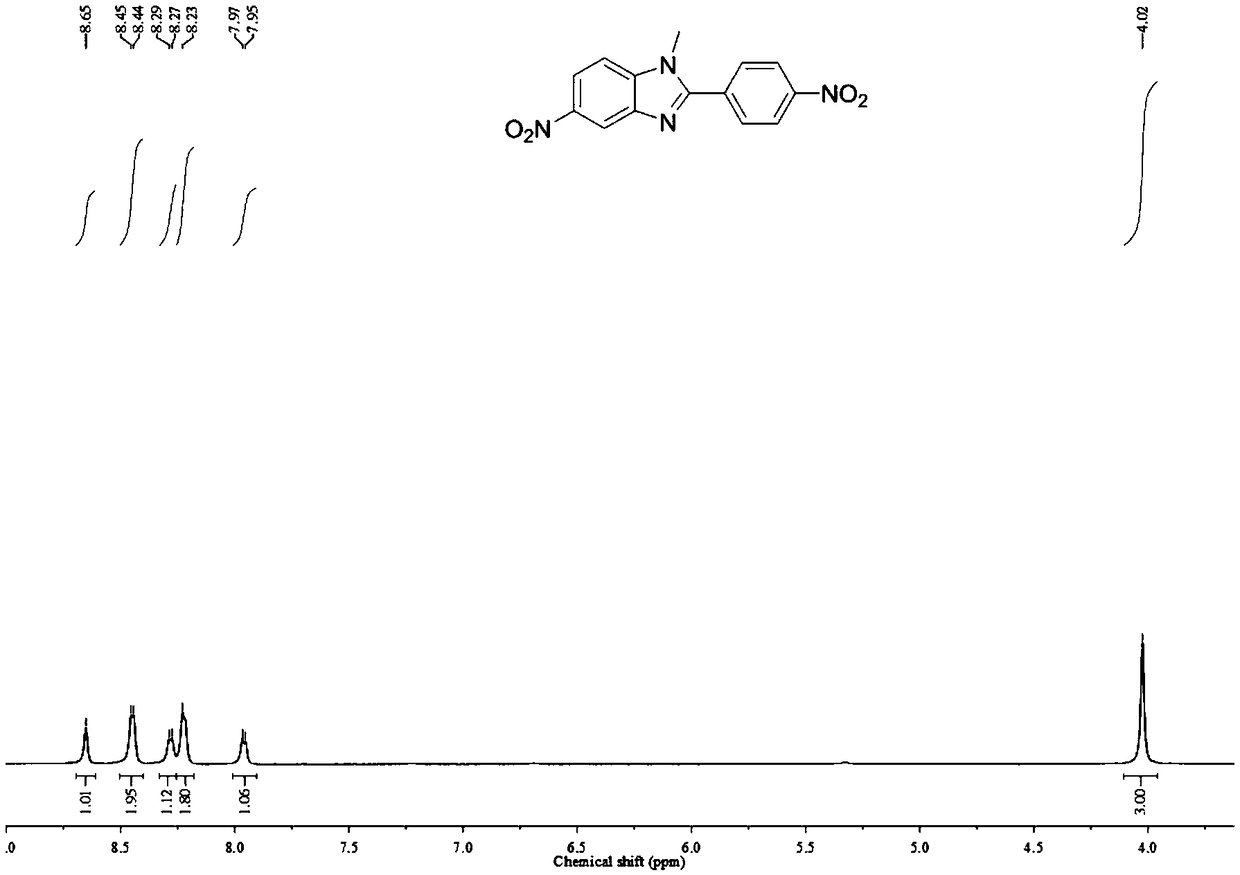
[0049] a. Add 50.0g N-methyl-4-nitrobenzene-1,2-diamine, 33.3g triethylamine and 500.0g tetrahydrofuran to the reaction flask, cool down to 5±1°C, add 61.0g p-nitro Benzoyl chloride was added dropwise in 2-3 hours, reacted at room temperature for 16 hours, and TLC confirmed that the reaction was complete. Add 1500.0g of water to the reaction system, filter under reduced pressure to obtain 90.0g of crude product, add 900.0g of acetic acid after drying, react at 100°C for 16h, TLC confirms that the reaction is complete, cool down to 0-5°C, and add 1400.0g water, dried under reduced pressure suction filtration, recrystallized from dimethylformamide, filtered, dried to obtain 74.3g 5-nitro-2-(4-nitrobenzene)-1-methylbenzimidazole, yield : 83.3%, confirm...
Embodiment 2
[0052] In this example, the specific structure of N-substituted benzimidazole diamine is as follows:
[0053]
[0054] The preparation method of above-mentioned 5-amino-2-(4-aminobenzene)-1-phenylbenzimidazole is as follows:
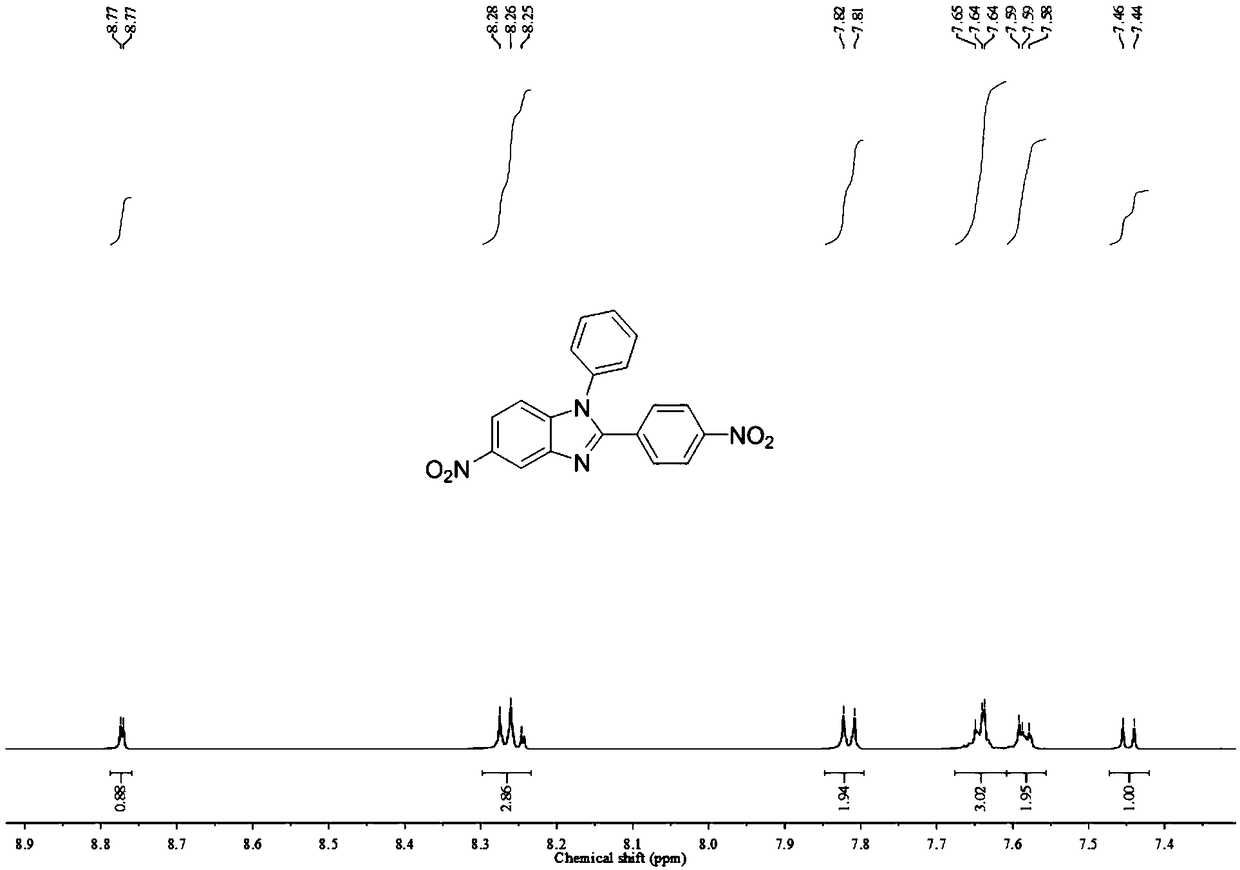
[0055]a. Add 50.0g N-phenyl-4-nitrobenzene-1,2-diamine, 19.0g pyridine and 500.0g dichloromethane to the reaction flask, cool down to 5±1℃, add 44.5g p-nitro Benzoyl chloride was added dropwise in 2-3 hours, reacted at room temperature for 16 hours, and TLC confirmed that the reaction was complete. Add 1500.0g of water to the reaction system, filter under reduced pressure to obtain 78.0g of crude product, add 700.0g of acetic acid after drying, react at 80°C for 16h, TLC confirms that the reaction is complete, cool down to 0-5°C, and add 1400.0g water, dried under reduced pressure suction filtration, recrystallized from dimethylformamide and water, filtered, dried to obtain 67.0g 5-nitro-2-(4-nitrobenzene)-1-phenylbenzimidazole, The yield was 85.3%,...
Embodiment 3
[0058] In this example, the specific structure of N-substituted benzimidazole diamine is as follows:
[0059]
[0060] The preparation method of above-mentioned 5-amino-2-(4-aminobenzene)-1-(2-fluorophenyl)-phenylbenzimidazole is as follows:
[0061] a. Add 50.0g N-(2-fluorophenyl)-4-nitrobenzene-1,2-diamine, 22.5g triethylamine and 500.0g toluene to the reaction flask, cool down to 5±1℃, add dropwise 41.4g of p-nitrobenzoyl chloride was added dropwise within 2 to 3 hours, reacted at room temperature for 16 hours, and TLC confirmed that the reaction was complete. Add 1500.0g of water to the reaction system, filter under reduced pressure to obtain 75.0g of crude product, add 700.0g of sulfuric acid after drying, react at 120°C for 16h, TLC confirms that the reaction is complete, cool down to 0-5°C, and pour into the reaction system 2000.0g of water, dried under reduced pressure suction filtration, recrystallized from dimethylacetamide, filtered, and dried to obtain 65.0g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com