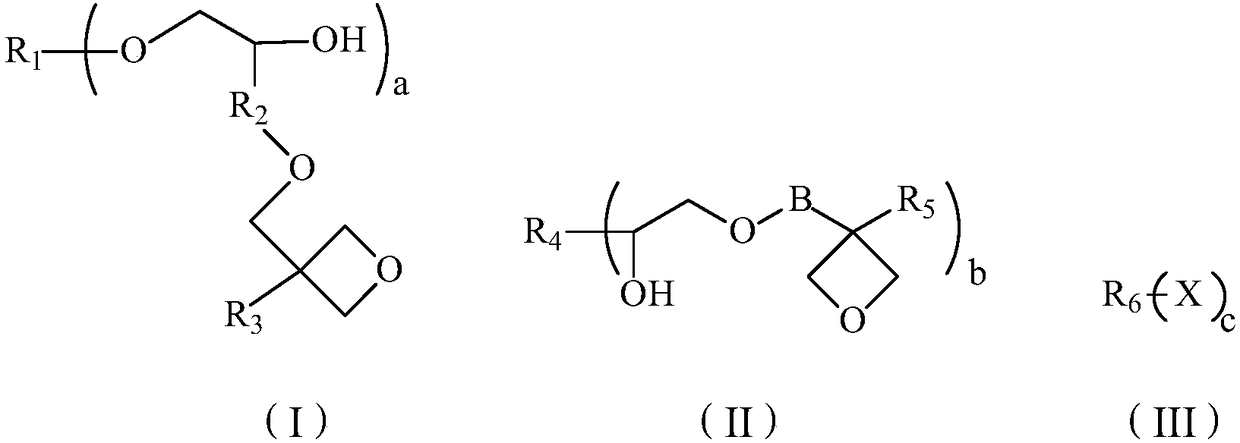
Oxetane compound and preparation method thereof
A technology for oxetane compounds, which is applied in the field of oxetane compounds and their preparation, can solve the problems of low selectivity, high viscosity, and limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
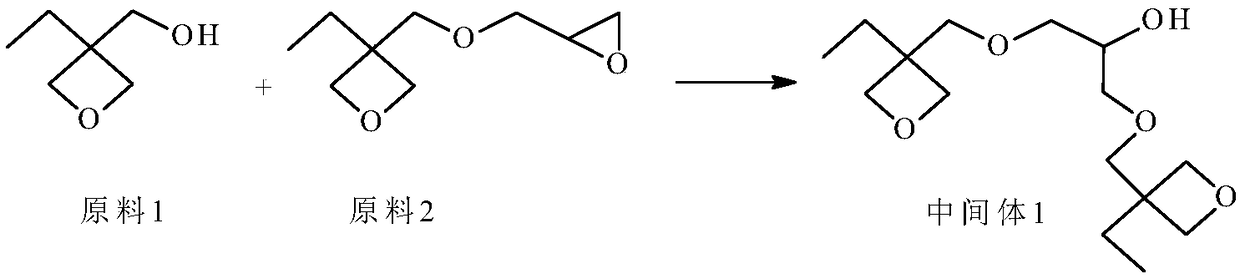
Embodiment 1
[0057] Preparation of Intermediate 1
[0058]
[0059] Add 58g (0.5mol) of raw material 1, 4g (0.1mol) of sodium hydroxide and 100g of toluene to a 250ml four-necked flask equipped with a stirring device, a thermometer, and a reflux condenser in sequence, stir and heat up to 80°C, and dropwise add 86g (0.5mol) of mol) raw material 2, after 1.5h dropwise addition, continue to stir the reaction, follow the gas phase until the content of raw material 1 no longer changes, stop heating, adjust the pH value to neutral with 5% hydrochloric acid solution, filter, add water to fully stir, let stand to separate The removal of the water layer was repeated twice, and the toluene was distilled off the organic layer under reduced pressure to obtain 136 g of a light yellow liquid.
[0060] The structure of intermediate 1 was determined by GC-MS and 1 H-NMR confirmed.
[0061] MS(m / e): 289(M+1);
[0062] 1 H-NMR (CDCl 3 , 500MHz): δ0.96(6H, m), δ1.25(4H, m), δ2.01(1H, d), δ3.29(4H, s)...
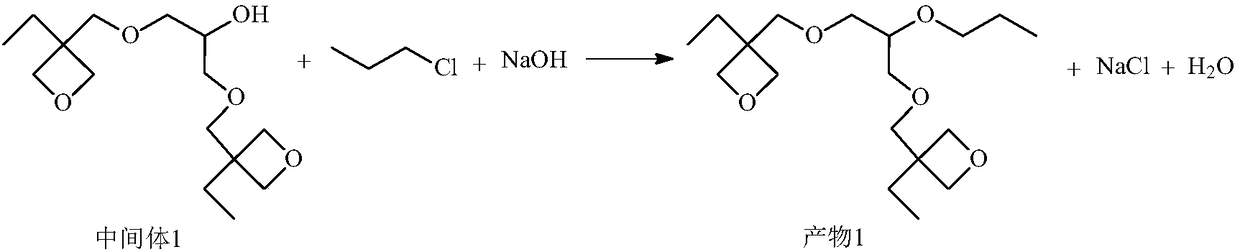
Embodiment 2
[0070] Preparation of product 2
[0071]
[0072] Add 144g (0.5mol) intermediate 1,44g (0.25mol) p-dichlorobenzyl, 20g (0.5mol) sodium hydroxide and 150ml toluene successively in the 500ml four-necked flask that stirrer, thermometer, reflux condenser are housed, Reflux reaction, follow the gas phase until the intermediate 1 completely disappears, after the reaction, add water to fully stir, let it stand, separate the water layer, repeat twice, distill the organic layer under reduced pressure, remove toluene, and finally obtain 165g of viscous liquid.
[0073] The structure of product 2 was determined by GC-MS and 1 H-NMR confirmed.
[0074] MS(m / e): 679(M+1);
[0075] 1 H-NMR (CDCl 3 , 500MHz): δ0.96(12H, m), δ1.25(8H, m), δ3.29-3.51(18H, m), δ4.63-4.65(20H, m), δ7.12(4H, d).
Embodiment 3
[0077] Preparation of Intermediate 2
[0078]
[0079] Add 58g (0.5mol) of raw material 3, 4g (0.1mol) of sodium hydroxide and 100g of toluene in sequence to a 500ml four-necked flask equipped with a stirring device, a thermometer, and a reflux condenser, stir and heat up to 80°C, and dropwise add 85g of (0.25mol) toluene solution of raw material 4, after 1.5h dropwise addition, continue stirring reaction, gas phase tracking until the content of raw material 3 no longer changes, stop heating, adjust pH value to neutral with 5% hydrochloric acid solution, filter, add water fully Stir, stand still to separate the water layer, repeat twice, distill the organic layer under reduced pressure to remove toluene, and obtain 136 g of light yellow liquid.
[0080] The structure of intermediate 2 was determined by GC-MS and 1 H-NMR confirmed.
[0081] MS(m / e): 573(M+1);
[0082] 1 H-NMR (CDCl 3 , 500MHz): δ0.96(6H, m), δ1.25(4H, m), δ1.67(6H, s), δ2.01(2H, s), δ3.29-δ3.52(8H, m),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com