Electrolyte solution to detect hexavalent chromium via mercury film electrode and method to detect hexavalent chromium via electrolyte solution
An electrolyte solution, a technology using mercury film, applied in the direction of material electrochemical variables, etc., can solve the problems of complexity, mercury film falling off, cumbersome and other problems, and achieve the effect of reducing the dissolution peak current value, improving sensitivity and reducing time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] 1) Take 5 clean 100mL volumetric flasks, add 10mL of the above electrolyte solution, respectively pipette 20μL, 40μL, 60μL, 80μL, 100μL of 100mg / L hexavalent chromium standard solution into 5 volumetric flasks, dilute to Scale, prepare 20μg / L, 40μg / L, 60μg / L, 80μg / L, 100μg / L series of standard solutions, respectively pipette 20mL into the measuring cup.
[0077] 2) Connect the three-electrode system consisting of mercury film electrode, reference electrode and platinum electrode to the portable heavy metal meter.
[0078] 3) Put the three-electrode system into the electrolytic cup containing 20μg / L, 40μg / L, 60μg / L, 80μg / L, 100μg / L serial standard solutions in sequence. Select the "Calibrate" button to calibrate each point in turn, and save the calibration data after the 5-point calibration is completed.
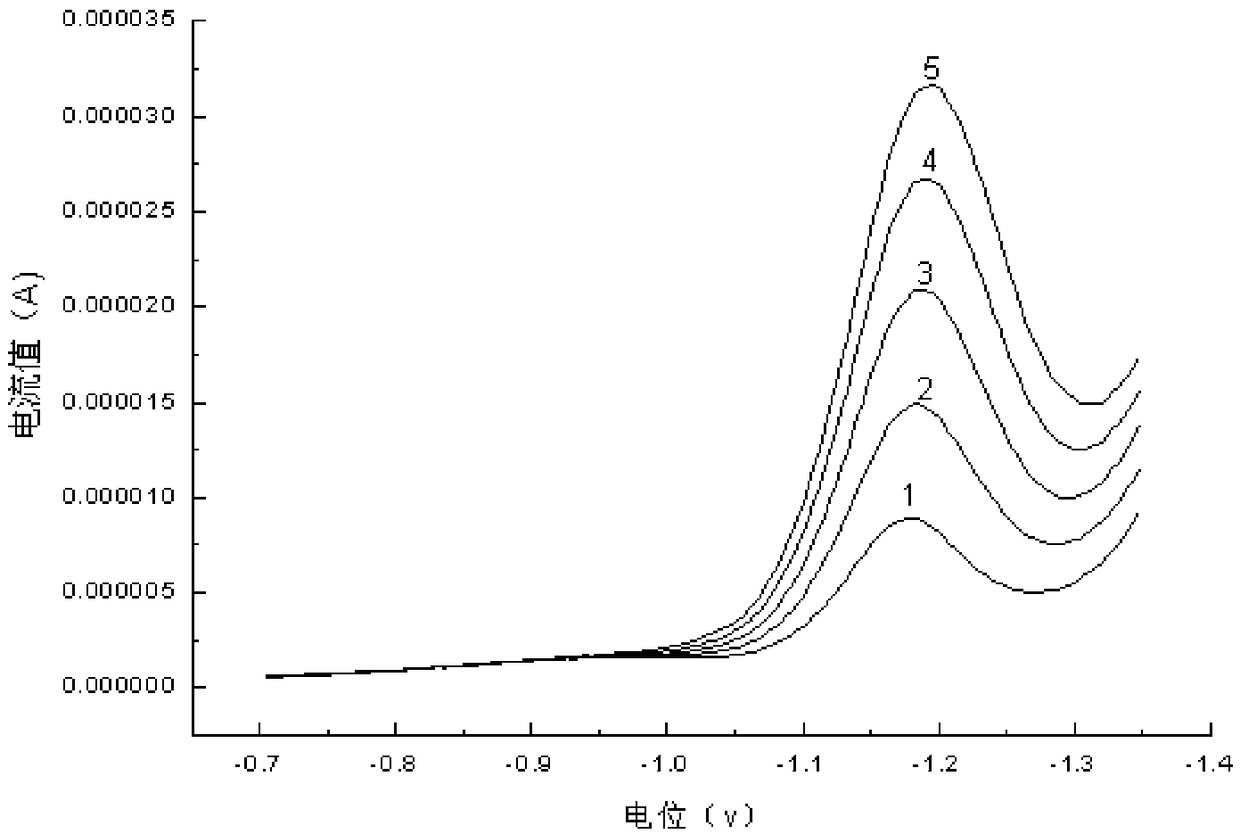
[0079] attached figure 1 It is the superposition of the peak patterns of the series of standard solutions in Example 1. The curves of different concentrations in the...
Embodiment 2
[0081] 1) Take a clean 200mL volumetric flask, add 20mL of the above electrolyte solution, pipette 100μL of 100mg / L hexavalent chromium standard solution into the volumetric flask, dilute to the mark, and prepare a 50μg / L hexavalent chromium standard solution, pipette 20mL into a measuring cup.
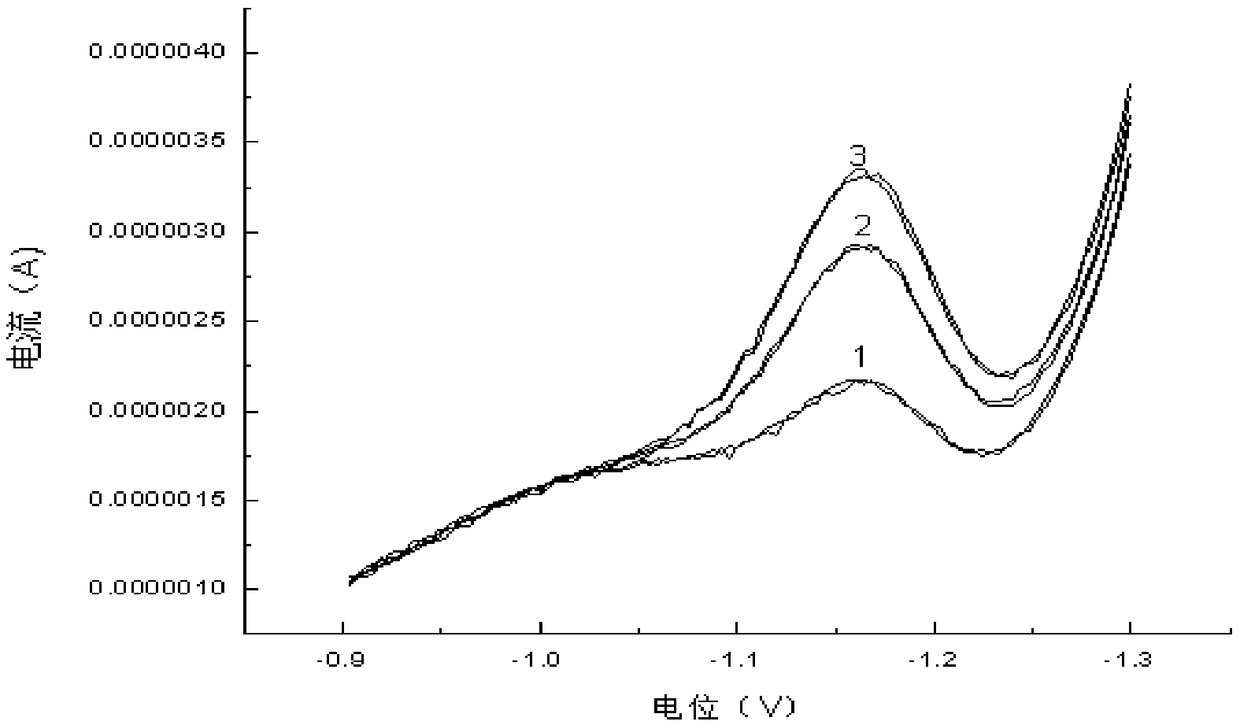
[0082] 2) Put the three-electrode system in Example 1 into the 50 μg / L hexavalent chromium standard solution, select the "measurement" button, and measure the solution for 6 consecutive times. The six measurement results were: 48.5μg / L, 50.4μg / L, 49.0μg / L, 49.0μg / L, 50.9μg / L, 49.3μg / L, and the relative standard deviation was 1.87%.
Embodiment 3
[0084] 1) Take a clean 100mL volumetric flask, add 10mL of the above electrolyte solution, dilute to the mark, mix well, pipette 20mL into the measuring cup for later use.
[0085] 2) Accurately pipette 40 μL of 1 mg / L hexavalent chromium standard solution into the measuring cup of 1), the theoretical value is 2 μg / L.
[0086] 3) Put the three-electrode system into the measuring cup and start the standard addition test. Add 60 μL of 1 mg / L hexavalent chromium standard solution to the same cup solution twice in a row. After the measurement, the instrument directly reads the result as 2.58 μg / L, close to the theoretical value of 2μg / L.
[0087] It can be seen from the above examples that hexavalent chromium Cr(VI) is first reduced to trivalent chromium Cr(III) on the surface of the working electrode of the pre-mercury-plated film, and DTPA present in the solution is adsorbed on the surface of the mercury film, and reacts with trivalent chromium Cr(III). (Ⅲ) Form complex Cr(Ⅲ)-D...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com