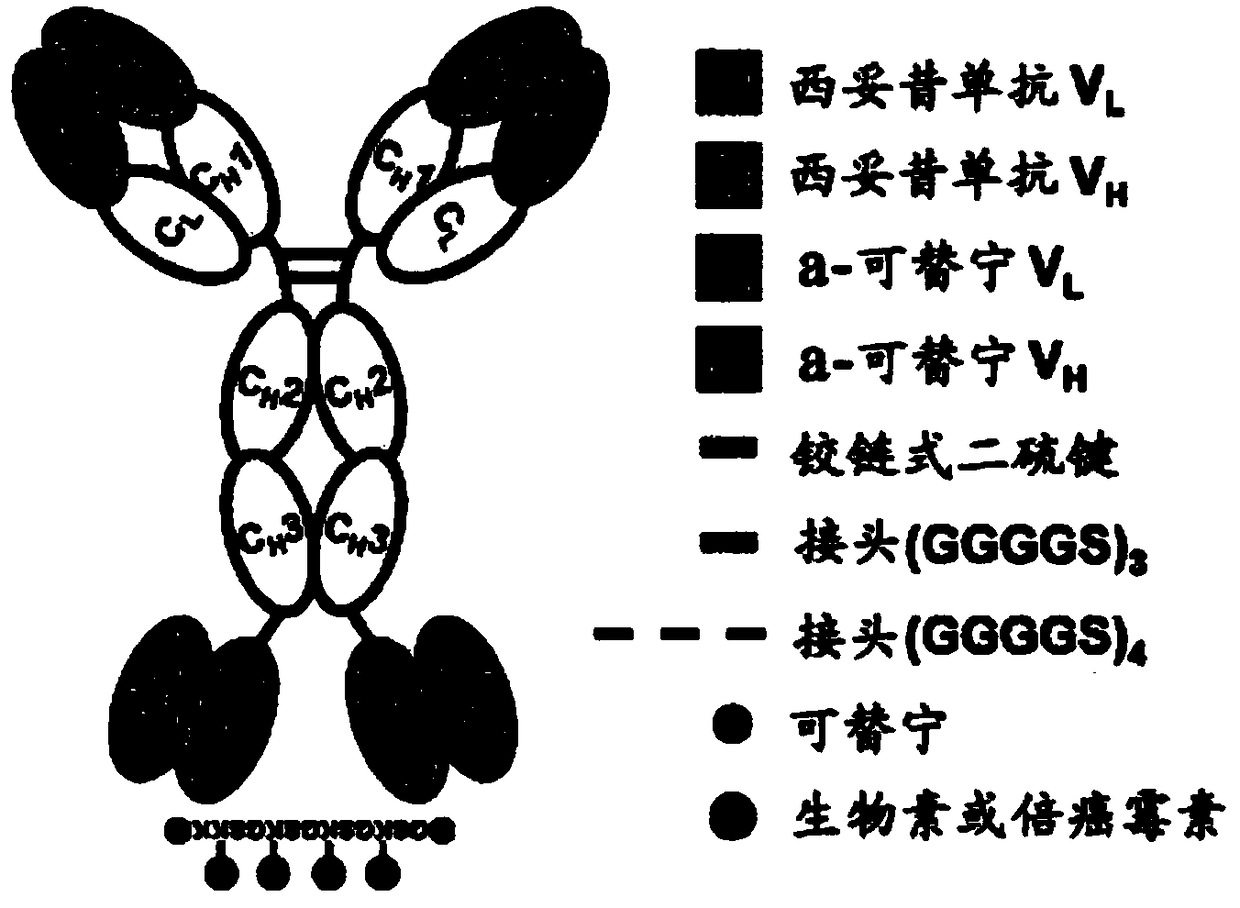
Antibody-drug composite platform using bispecific antibody
A bispecific antibody, antibody drug technology, applied in the direction of antibodies, drug combinations, anti-tumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Cell Culture
[0050] A549 (human lung adenocarcinoma) cells were obtained from a Korean cell line bank, and supplemented with 10% heat-inactivated fetal bovine serum (GIBCO, Grand Island, NY, USA), 100 U / mL penicillin, and 100 μg / mL chain Mycin RPMI-1640 medium (Welgene, Seoul, South Korea) containing 5% CO 2 grow in a moist atmosphere. HEK293F cells (Invitrogen, Carlsbad, CA, USA) were cultured in vented Erlenmeyer tissue culture flasks (Corning Inc., NY, USA) in FreeStyle® containing 100 U / mL penicillin and 100 μg / mL streptomycin. TM In 293Expression medium (GIBCO), in a rotary shaking incubator (Minitron, INFORS HT, Bottmingen, Switzerland) at 135rpm, at 37°C, containing 7% CO 2 grow in a 70% humid atmosphere.
Embodiment 2
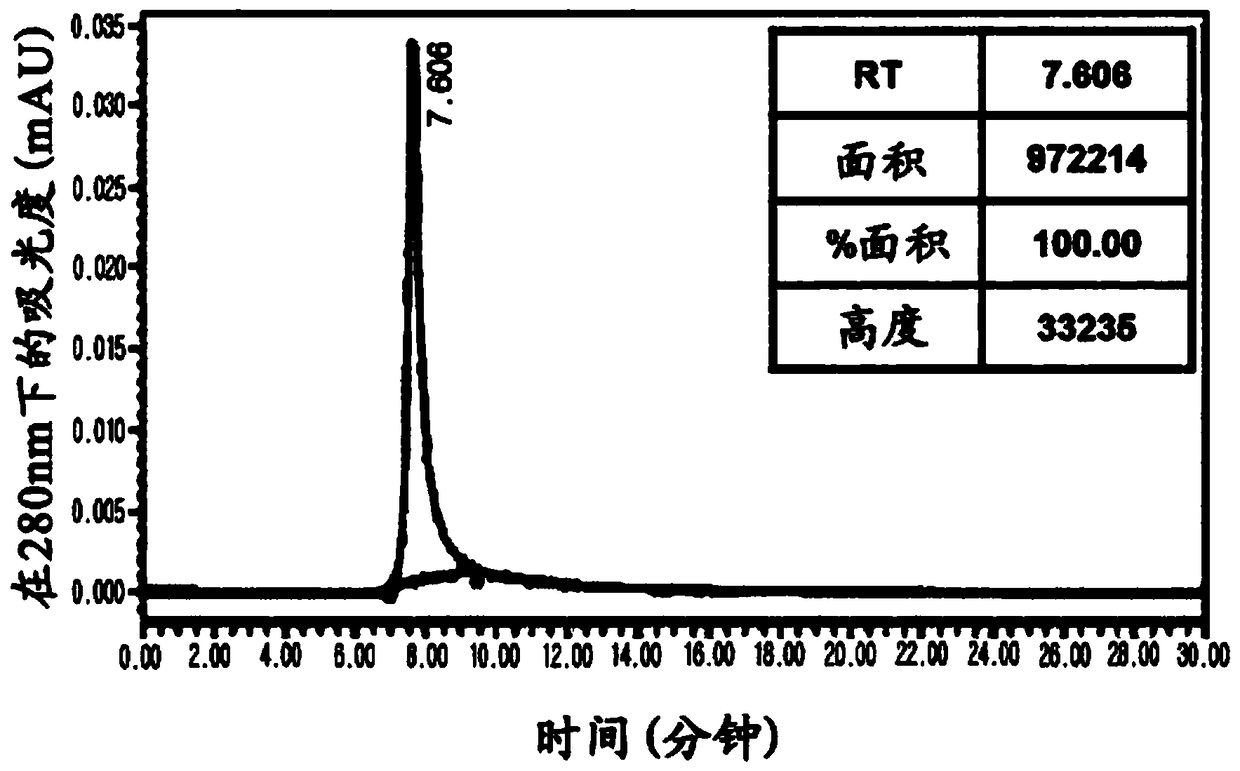
[0051] Example 2: Construction and purification of bispecific cetuximab × anti-cotinine antibody
[0052] In order to construct a bispecific cetuximab × anti-cotinine antibody expression vector, chemically synthesized cetuximab light chain and cetuximab heavy chain-linker (Gly-Gly-Gly-Gly-Ser ) 3 - Anti-cotinine single-chain variable fragment (scFv) gene (Genscript, Piscataway, NJ, USA). Restriction sites AgeI and XbaI were inserted at the 5' and 3' ends of the gene encoding the cetuximab light chain, respectively. Additional restriction sites NheI and BsiWI were inserted at the 5' end of the gene encoding the cetuximab heavy chain and at the 3' end of the anti-cotinine scFv gene. Light and heavy chain-linker-anti-cotinine-scFv were subcloned into mammalian expression vectors designed for secretion of recombinant proteins as described in the following methods: Park S, Lee DH, Park JG, Lee YT, Chung J. A sensitive enzyme immunoassay for measuring cotinine in passive smokers....
Embodiment 3
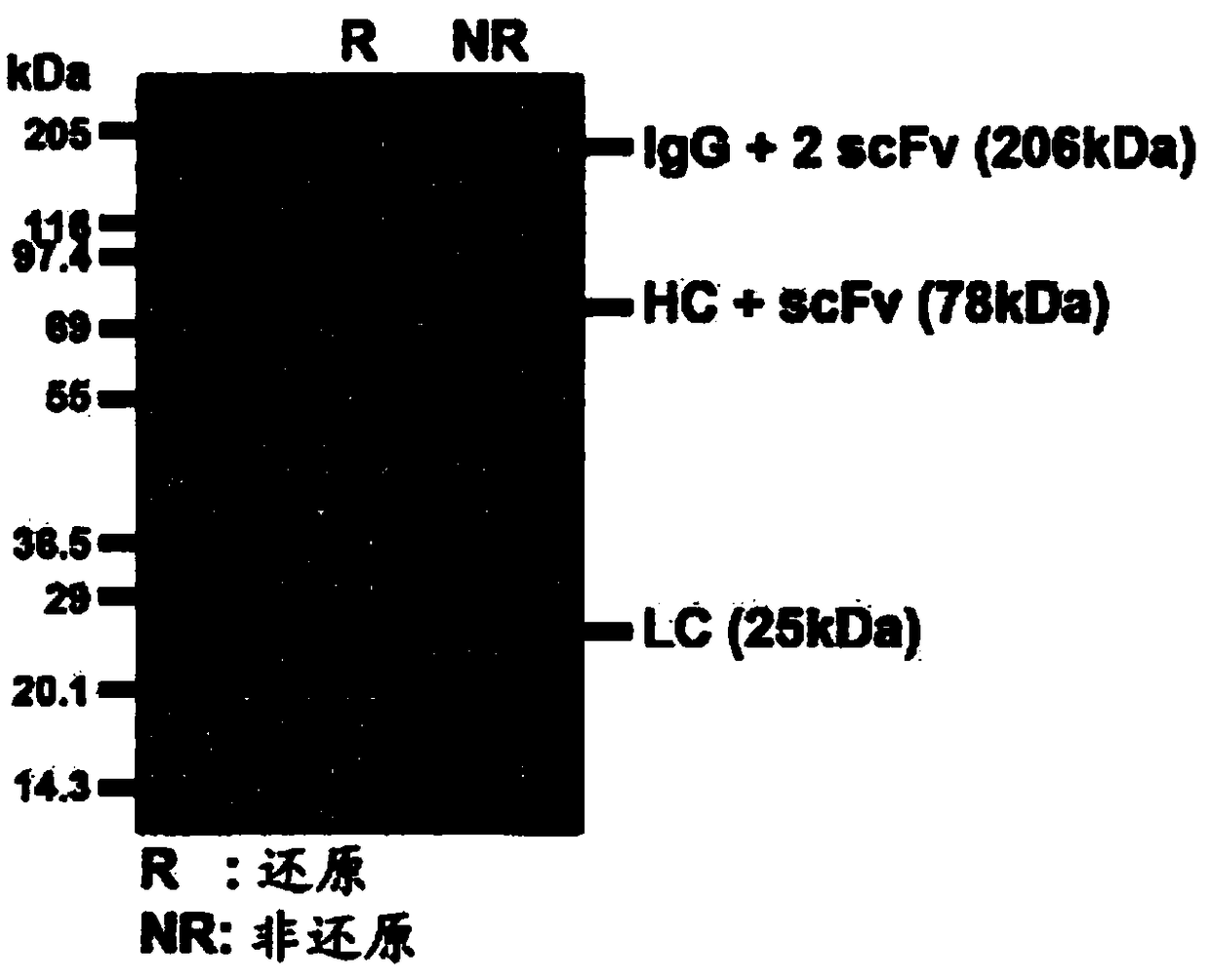
[0054] Embodiment 3: sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
[0055] Analysis was performed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using NuPage 4% to 12% Bis-Tris gels (Invitrogen) according to the manufacturer's instructions. Gels were stained with Coomassie brilliant blue R-250 (Amresco, Colon, OR, USA).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com