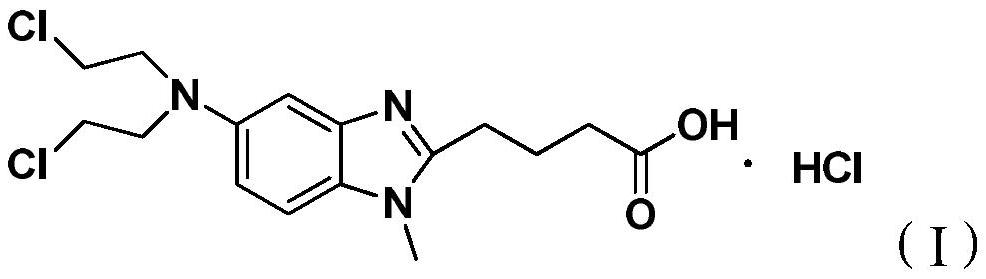
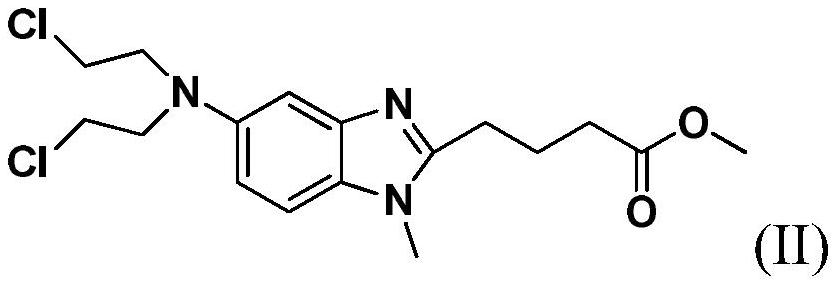
A kind of preparation method of bendamustine hydrochloride crude product
A technology of bendamustine hydrochloride crude product and concentrated hydrochloric acid, applied in the field of medicine, can solve the problems of unfavorable mass industrial production, increase production cost, and increase of impurities in the concentration process, and achieve high yield and product purity, and little environmental pollution. , the effect of easy reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 10 g of 4-{5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}-butyric acid methyl ester to 100 g of 36% concentrated hydrochloric acid After stirring and dissolving, add 0.5g of activated carbon, stir and raise the temperature to 95±5°C for 4 hours, lower to 15-30°C, filter, add 450g of purified water to the filtrate, continue to cool down to 2°C, stir for 1.5h, then filter, The filter cake was rinsed once with 10 g of ice-purified water and 10 g of ice-based acetone, and the filter cake was vacuum-dried at 40-50° C. for 12 hours to obtain 7.8 g of a white solid with a yield of 76.3%. The HPLC chromatographic purity of the sample was 99.43%. The content of {5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}-butyric acid methyl ester is 0.18%, and the maximum other impurities are 0.10%.
[0028] HPLC purity detection method
[0029] Take about 20mg of this product, put it into a 25ml volumetric flask, add a small amount of methanol to dissolve ...
Embodiment 2
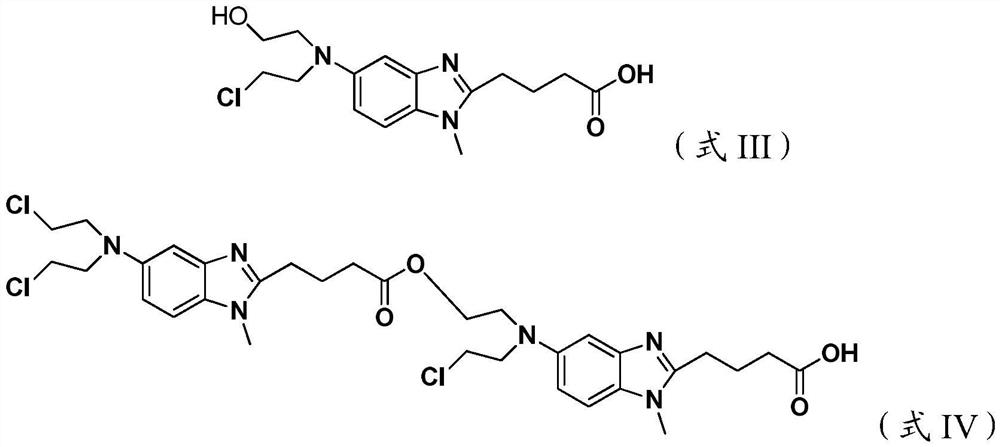
[0033] Add 10 g of 4-{5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}-butyric acid methyl ester to 100 g of 36% concentrated hydrochloric acid After stirring and dissolving, add 0.5g of activated carbon, stir and raise the temperature to 95±5°C for 4 hours, lower to 15-30°C, filter, add 400g of saturated sodium chloride solution to the filtrate, continue to cool down to 3°C, and stir for 1.5h , filtered, the filter cake was rinsed once with 10 g of ice-purified water and 10 g of ice-based acetone, and the filter cake was vacuum-dried at 40-50 ° C for 12 hours to obtain 8.3 g of a white solid, with a yield of 81.2%, and a sample chromatographic purity of 99.24%. 4-{5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}-butyric acid methyl ester content 0.39%, formula III compound content 0.05% , the content of the compound of formula IV is 0.07%, the maximum other impurities are 0.14%, and the total impurities are 0.76%. HPLC purity detection condition is ...
Embodiment 3
[0035]Add 10 g of 4-{5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}-butyric acid methyl ester to 100 g of 36% concentrated hydrochloric acid Add 0.5g of activated carbon after stirring and dissolving, stir and heat up to 95±5°C for 4h, lower to 15-30°C, filter, add 420g of saturated potassium chloride solution to the filtrate, continue to cool down to -5°C, and stir for 1.5h After filtering, the filter cake was rinsed once with 10 g of ice purified water and 10 g of ice acetone respectively, and the filter cake was vacuum-dried at 40-50 °C for 12 hours to obtain 8.0 g of white solid, yield 78.3%, sample chromatographic purity 99.20% , 4-{5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}-butyric acid methyl ester content 0.48%, other maximum single hetero 0.11 %. HPLC purity detection condition is the same as embodiment 1
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com