Metronidazole and sodium chloride injection as well as preparation method and application thereof
A technology of metronidazole sodium chloride and nidazole sodium chloride, which is applied in the direction of pharmaceutical formulas, medical preparations without active ingredients, medical preparations containing active ingredients, etc., can solve the problem of metronidazole sodium chloride injection Poor stability and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The second aspect of the present invention provides a kind of preparation method of metronidazole sodium chloride injection, and the method comprises the following steps:
[0030] (1) Dissolve metronidazole, citric acid and sodium dihydrogen phosphate in water for injection, then add activated carbon, filter and decarbonize after stirring, to obtain intermediate solution A;
[0031] (2) dissolving sodium chloride in water for injection, then adding activated carbon, stirring and filtering to decarbonize to obtain intermediate solution B;
[0032] (3) Mix the intermediate liquid A and intermediate liquid B, then add disodium hydrogen phosphate to adjust the pH value to 4.7-7.0, add activated carbon, heat and boil, cool and filter for decarbonization in sequence, and pack under sterile conditions potting,
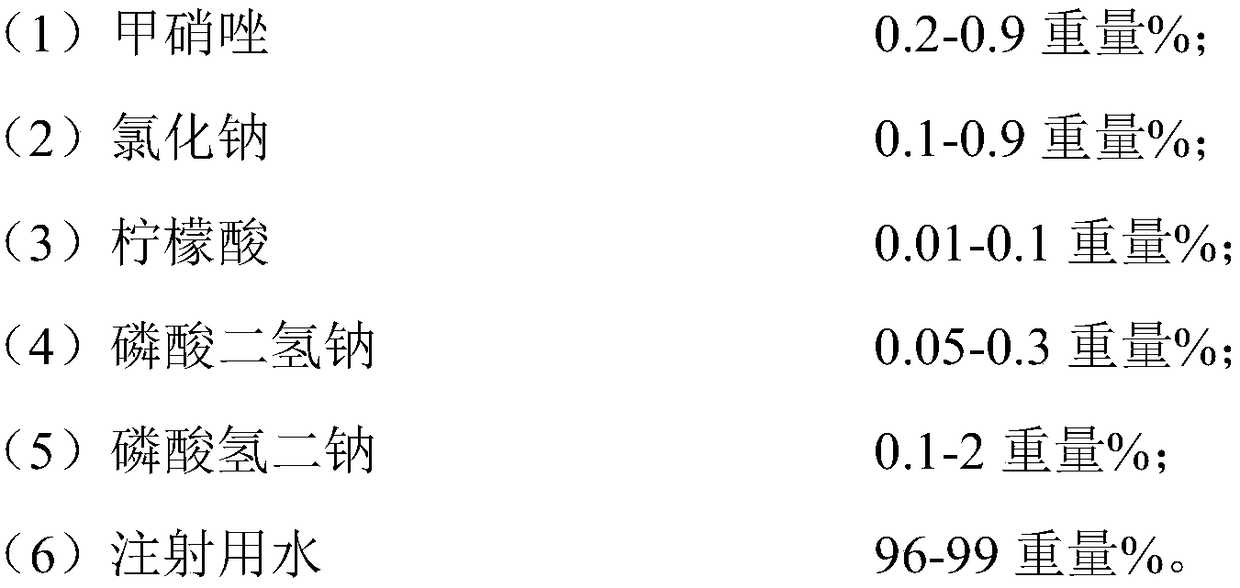
[0033] Wherein, the feeding ratio of metronidazole, sodium chloride, citric acid, sodium dihydrogen phosphate, disodium hydrogen phosphate and water for injection is ...
Embodiment 1
[0050] (1) Add 20L of water for injection into the first concentrated tank, then add 250g of metronidazole (D90≤6.35μm), 37.5g of citric acid and 75g of sodium dihydrogen phosphate, then add activated carbon, stir for 15min, and use 0.22μm The microporous membrane is filtered and decarbonized to obtain the intermediate solution A;
[0051] (2) Add 20L of water for injection into the second concentrated preparation tank, then add 400g of sodium chloride, then add activated carbon, stir for 15min, and filter and decarbonize with a 0.22 μm microporous membrane to obtain intermediate solution B;
[0052] (3) Mix the intermediate liquid A and intermediate liquid B, and transfer to the dilute preparation tank, set the volume to 50 L with water for injection, then add 100 g of disodium hydrogen phosphate to adjust the pH value to 6.2, then add activated carbon, and stir for 15 min. And use a 0.22 μm microporous membrane to filter and decarbonize, and pack and seal under sterile condi...
Embodiment 2
[0054] (1) Add 20L of water for injection into the first concentrated tank, then add 250g of metronidazole (D90≤6.35μm), 50g of citric acid and 125g of sodium dihydrogen phosphate, then add activated carbon, stir for 15min, and use 0.22μm The microporous membrane is decarbonized by filtration to obtain the intermediate solution A;
[0055] (2) Add 20L of water for injection into the second concentrated tank, then add 450g of sodium chloride, then add activated carbon, stir for 15min, and use a 0.22μm microporous membrane to filter and decarbonize to obtain intermediate solution B;
[0056] (3) Mix the intermediate solution A and the intermediate solution B, and transfer to the dilute tank, set the volume to 50L with water for injection, then add 100g of disodium hydrogen phosphate to adjust the pH value to 4.7, then add activated carbon, stir for 15min, And use a 0.22 μm microporous membrane to filter and decarbonize, and pack and seal under sterile conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com