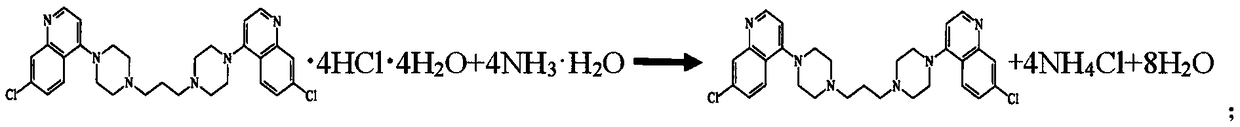High-yield piperaquine preparation method
A high-yield, piperaquine technology, applied in the field of preparation of anti-malarial drugs, can solve the problems of low purity of piperaquine, low total yield, high cost, etc., achieve the elimination of nausea and vomiting side effects, reduce the total amount of medication, and reduce production costs low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] First, calculate the amount of raw materials according to the obtained amount of piperaquine theory of 535.56g (i.e. the amount of 1mol); specifically, add 15071g of purified water in a 40L dissolution tank, heat to 70°C, and then weigh 753.56g of piperaquine hydrochloride (i.e. 1mol) into hot water, stirred, then heated to 85 ° C to completely dissolve piperaquine hydrochloride, and filtered to obtain piperaquine hydrochloride solution.
[0025] Next, take by weighing the ammoniacal liquor 420g (being the amount of 3mol) that concentration is 25%, slowly join in the above-mentioned obtained piperaquine hydrochloride solution, stir while adding, and reaction temperature is controlled at 70~80 ℃, after adding Continue to stir for 4 minutes, after the reaction, the piperaquine precipitate can be obtained by centrifugal filtration.
[0026] Finally, the obtained piperaquine precipitate was washed repeatedly with purified water twice until the residual hydrochloride in the ...
Embodiment 2
[0028] First, calculate the amount of raw materials according to the obtained amount of piperaquine theory of 535.56g (that is, the amount of 1mol); specifically, add 22607g of purified water to a 40L dissolution tank, heat to 70°C, and then weigh 753.56g of piperaquine hydrochloride (that is, 1mol amount) into hot water, stirred, then heated to 90 ° C to completely dissolve piperaquine hydrochloride, and filtered to obtain piperaquine hydrochloride solution.
[0029] Next, take by weighing the ammoniacal liquor 560g (being the amount of 4mol) that concentration is 25%, slowly join in the above-mentioned obtained piperaquine hydrochloride solution, stir while adding, and reaction temperature is controlled at 70~80 ℃, after adding Continue to stir for 4 minutes, after the reaction, the piperaquine precipitate can be obtained by centrifugal filtration.
[0030] Finally, the obtained piperaquine precipitate was repeatedly washed with purified water for 3 times until the residual ...
Embodiment 3
[0032] First, calculate the amount of raw materials according to the obtained amount of piperaquine theory of 535.56g (that is, the amount of 1mol); specifically, add 30142g of purified water to a 40L dissolution tank, heat to 70°C, and then weigh 753.56g of piperaquine hydrochloride (that is, 1mol amount) into hot water, stirred, then heated to 95 ° C to completely dissolve piperaquine hydrochloride, and filtered to obtain piperaquine hydrochloride solution.
[0033] Next, take by weighing the ammoniacal liquor 700g (being the amount of 5mol) that concentration is 25%, slowly join in the above-mentioned obtained piperaquine hydrochloride solution, stir while adding, and reaction temperature is controlled at 70~80 ℃, after adding Continue to stir for 4 minutes, after the reaction, the piperaquine precipitate can be obtained by centrifugal filtration.
[0034] Finally, the obtained piperaquine precipitate was repeatedly washed with purified water for 4 times until the residual ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com