Preparation method of 4,5-diphenyl substituted triazole compound
A technology of diphenyl and compound, which is applied in the field of preparation of 4,5-diphenyl substituted triazole compounds, and can solve problems such as harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 biological carbon load base
[0021] (1) Take Ginkgo biloba (200g), wash and dry to constant weight, 2 In the presence of the presence, the temperature was raised to 800°C at a rate of 10°C / min, and after carbonization for 3 hours, the temperature was lowered to room temperature to obtain biochar;
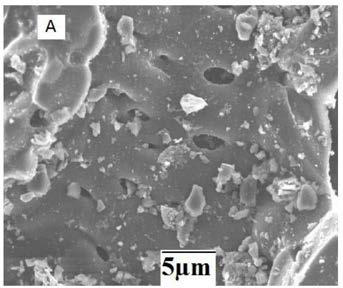
[0022] (2) Get the biochar (10g) obtained in step (1) and soak it with sodium hydroxide solution (6mol / L, 100mL) for 12 hours, wash with water, and dry to obtain the biochar-loaded alkali (hereinafter referred to as product A, figure 1 ).
Embodiment 2
[0024] Get the biochar (10g) obtained in step (1) of Example 1 and soak it with potassium hydroxide solution (4mol / L, 150mL) for 12 hours, wash and dry to obtain the biochar-loaded alkali (hereinafter referred to as product B, and figure 1 consistent).
Embodiment 3
[0025] Example 3 Preparation of 4,5-diphenyl-2H-1,2,3-triazole
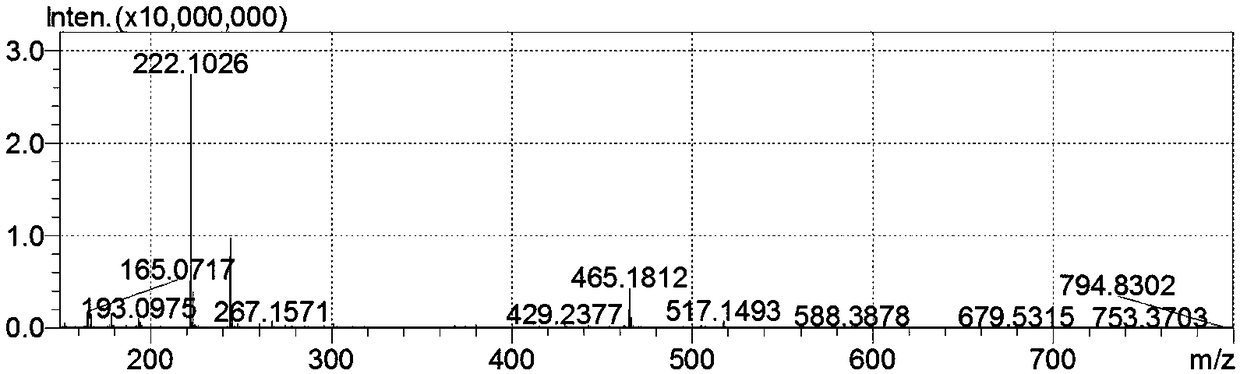
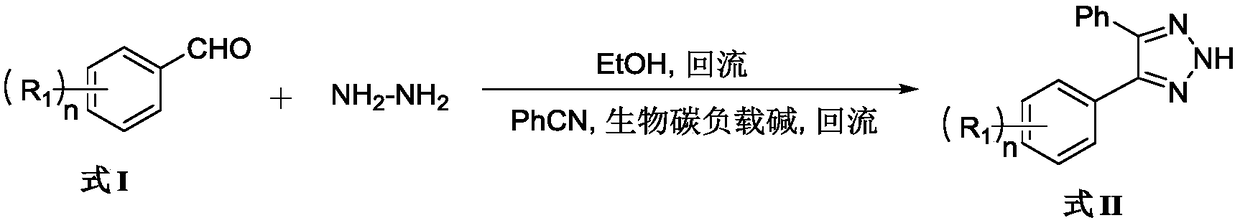
[0026] Dissolve benzaldehyde (2mmol) in absolute ethanol (20mL), add anhydrous hydrazine (2.4mmol), heat to reflux temperature and react for 6 hours, add benzonitrile (1.6mmol) and product A (20mg), continue After reflux reaction for 5 hours, product A was recovered by filtration. After the filtrate was concentrated, it was diluted with chloroform, washed with water and saturated sodium chloride successively, dried over anhydrous sodium sulfate, filtered and concentrated, and subjected to silica gel column chromatography (200-300 mesh silica gel ), using petroleum ether / ethyl acetate (15:1-10:1) as an eluent to obtain 329 mg of a white solid, which is 4,5-diphenyl-2H-1,2,3-triazole compound, The yield is about 92.9%. 1 H NMR (CDCl 3 ,400MHz), δ:12.82(br s,1H),7.54(d,J=3.3Hz,4H),7.38-7.36(m,6H). 13 C NMR (CDCl 3 ,100MHz), δ:142.8,130.4,128.7,128.6,128.3.HRMS (such as figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com