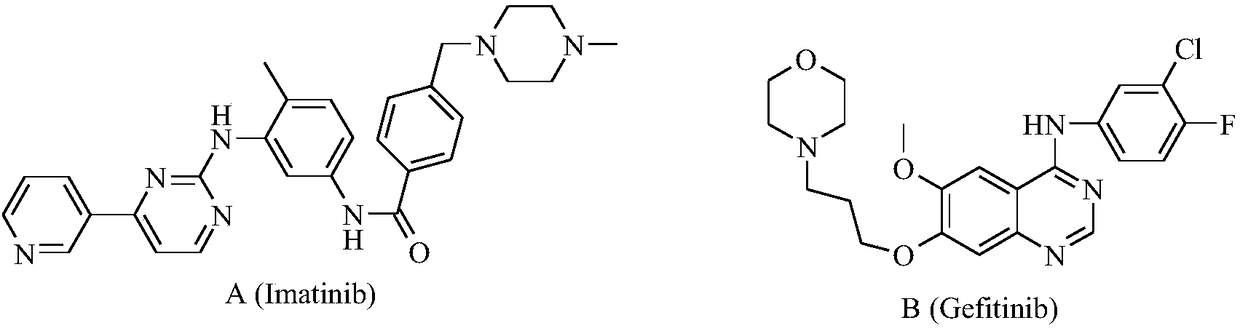
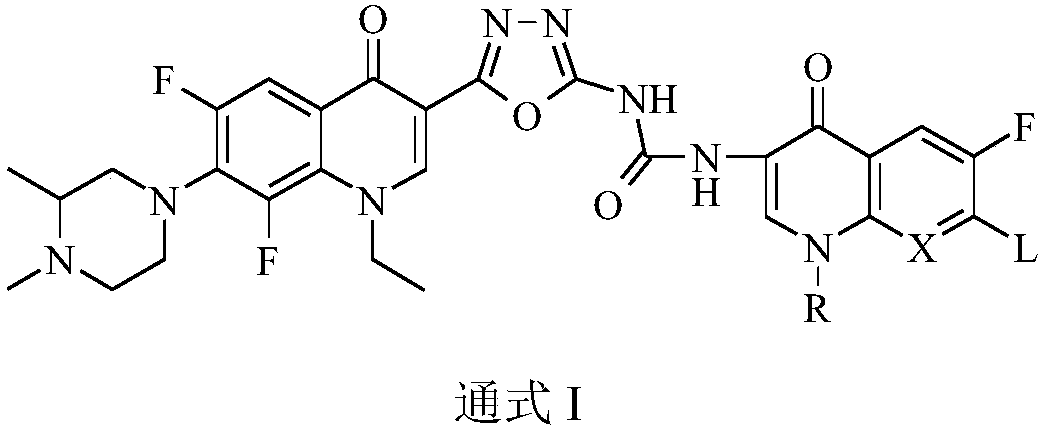
Bis-fluoroquinolone oxadiazole urea derivatives containing N-methyl gatifloxacin as well as preparation method and application of derivatives
A kind of technology of fluoroquinolone-based oxadiazole and gatifloxacin, which is applied in the fields of new drug discovery and innovative drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The general method for the preparation of fluoroquinolone hydroxamic acid (1″-18″) is as follows: take the aforementioned crude product of fluoroquinolone carboxylic acid imidazolamide (0.10 mol) and suspend it in 500 mL of pyridine, add 7.0 g to 35 g (0.1 to 0.50 mol) of hydroxylamine hydrochloride , stirred in a water bath at 60-75°C for 8.0-24.0 hours, cooled to room temperature, collected the solid by filtration, washed the solid with pyridine, dried it in vacuum at 60-70°C, and dispersed it in saturated sodium bicarbonate solution (500mL) again. Stir in a water bath at 65°C for 3 to 5 hours, collect the solid by filtration, wash with deionized water until the pH is 7.0, and dry to obtain a crude product. Crystallized to obtain analytically pure crystalline fluoroquinolone hydroxamic acid (1"-18").
[0046] The general method for the preparation of target compound bis-fluoroquinolone-based oxadiazole derivatives containing N-methylgatifloxacin: get each 1.0g of fluo...
Embodiment 1
[0050] 1-{2-[1-cyclopropyl-6-fluoro-8-methoxy-7-(3,4-dimethylpiperazin-1-yl)-quinolin-4(1H)-one- 3-yl]-1,3,4-oxadiazol-5-yl}-3-[6-fluoro-7-(4-methylpiperazin-1-yl)-8,1-(1,3 -oxypropyl)-quinolin-4(1H)-one-3-yl]-urea (I-1), its chemical structural formula is:
[0051]
[0052] The preparation method of the two-fluoroquinolone oxadiazuron of the present embodiment is: get ofloxacin hydroxamic acid (1 ") 1.0g (2.7 mmol) and suspend in 25mL acetonitrile, add CDI0.79g (4.9mmol), Stir at room temperature until the material is dissolved. Then add 1.16g (2.7mmol) of N-methylgatifloxacin C-3 oxadiazolamide II intermediate, and stir in a water bath at 55-60°C for 15 hours. Leave it overnight and filter the resulting solid , washed with acetonitrile. The crude product was recrystallized from a mixed solvent of DMF-ethanol to obtain a light yellow crystal (I-1), with a yield of 56%, m.p.214-216°C. 1 H NMR (400MHz, DMSO-d 6 )δ:11.65(brs,1H,NH), 9.47(s,1H,NH),9.32,9.16(2s,2H,2×2′-H),8....
Embodiment 2
[0054] (S)-1-{2-[1-cyclopropyl-6-fluoro-8-methoxy-7-(3,4-dimethylpiperazin-1-yl)-quinoline-4(1H )-keto-3-yl]-1,3,4-oxadiazol-5-yl}-3-[6-fluoro-7-(4-methylpiperazin-1-yl)-8,1- (1,3-oxopropyl)-quinoline-4(1H)-one-3-yl]-urea (I-1), its chemical structural formula is:
[0055]
[0056] The preparation method of the bis-fluoroquinolone oxadiazole of the present embodiment is: take levofloxacin hydroxamic acid (2 ") 1.0g (2.7 mmol) and suspend in 25mL acetonitrile, add CDI0.70g (4.3mmol), stir at room temperature until The material is dissolved. Then add 1.16g (2.7mmol) of N-methylgatifloxacin C-3 oxadiazolamine II intermediate, and stir in a water bath at 55-60°C for 10 hours. Place it overnight, collect the solid produced by filtration, and wash with acetonitrile The crude product was recrystallized from ethanol to obtain a light yellow crystal (I-2), with a yield of 46%, m.p.208-210°C. 1 H NMR (400MHz, DMSO-d 6 )δ: 11.66 (brs, 1H, NH), 9.50 (s, 1H, NH), 9.34, 9.18 (2s, 2H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com