Molecularly imprinted polymer with reversible covalent binding function and preparation method and application of molecularly imprinted polymer
A molecular imprinting and covalent binding technology, applied in the analysis of materials, material analysis by electromagnetic means, instruments, etc., can solve the problems of practicability and simple methods that have not yet been reported, and achieve stable template-monomer structure, good effect, Strong binding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
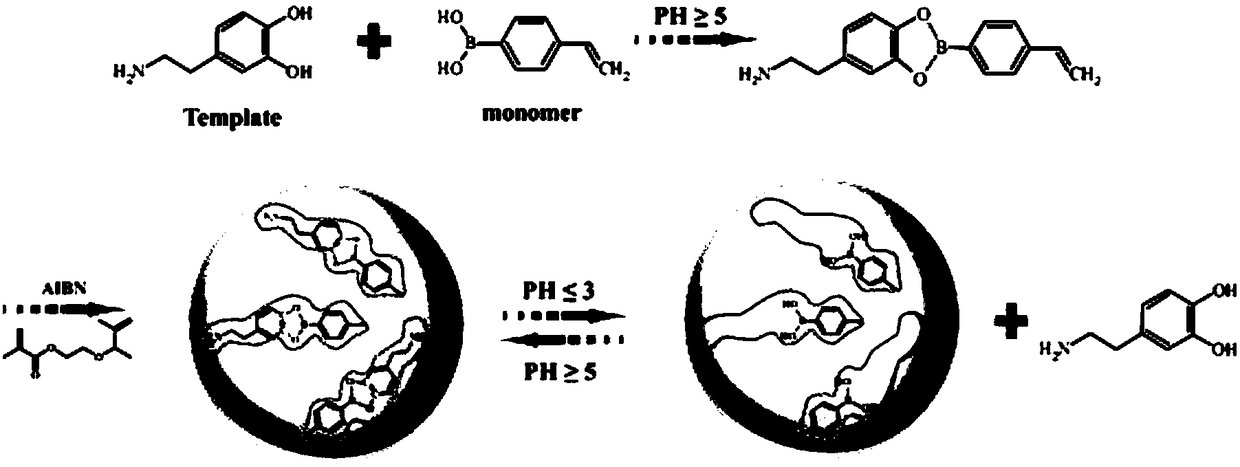
[0032] like figure 1 The present invention discloses a method for preparing a molecularly imprinted polymer with a reversible covalent binding function, and the steps of the method are as follows:
[0033] Synthesis of covalent-MIPs: template DA1mmol, functional monomer 4-vinylphenylboronic acid (VPBV) 2mmol, crosslinker ethylene glycol dimethacrylate (EGDMA) 2mmol and free radical initiator AIBN25mg were dissolved in acetonitrile (25mL) and DMF (15mL). The mixture was sonicated for 10 min to maintain homogeneity. Then use mild N 2 The solution was purged with air flow for 15 minutes and the 2 Seal under atmosphere. Finally, the flask was immersed in an oil bath at 70°C for 24 hours for polymerization.
[0034] Membrane plate removal: The template was removed by alternating solvent extraction with methanol / acetic acid (8 / 2, v / v) and hydrochloric acid solution (pH=2). Complete template removal was confirmed by ultraviolet absorption spectroscopy (UV). The resulting polym...
Embodiment 2
[0043] Synthesis of covalent-MIPs: template DA (1 mmol), functional monomer VPBV (2 mmol), EGDMA (10 mmol) and free radical initiator AIBN (20 mg) were dissolved in acetonitrile (45 mL) and DMF (15 mL). The mixture was sonicated for 10 min to maintain homogeneity. then use N 2 The solution was purged with gas for 15 min and the 2 Seal under atmosphere. Finally, the flask was immersed in an oil bath at 70°C for 24 hours for polymerization.
[0044] Membrane plate removal: The template was removed by solvent extraction in batch mode with methanol / acetic acid (8 / 2, v / v) and hydrochloric acid solution (pH=2). Complete template removal was confirmed by ultraviolet absorption spectroscopy (UV). The resulting polymer was then vacuum dried overnight at 40°C.
[0045] Wherein, the synthetic method of covalent-MIPs is as follows:
[0046] Template DA (1 mmol), functional monomer VPBV (2 mmol), EGDMA (10 mmol) and radical initiator AIBN (20 mg) were dissolved in acetonitrile (45 mL...
Embodiment 3
[0048] Synthesis of covalent-MIPs: template DA (1 mmol), functional monomer VPBV (2 mmol), EGDMA (10 mmol) and free radical initiator AIBN (20 mg) were dissolved in acetonitrile (45 mL) and DMF (15 mL). The mixture was sonicated for 10 min to maintain homogeneity. then use N 2 The solution was purged with gas for 15 min and the 2 Seal under atmosphere. Finally, the flask was immersed in an oil bath at 80°C for 24 hours for polymerization.
[0049] Membrane plate removal: The template was removed by solvent extraction in batch mode with methanol / acetic acid (8 / 2, v / v) and hydrochloric acid solution (pH=2). Complete template removal was confirmed by ultraviolet absorption spectroscopy (UV). The resulting polymer was then vacuum dried overnight at 40°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com