Benzimidazole derivative and preparation method and application as medicine thereof
A drug, benzo technology, applied in the field of pharmacology, can solve problems such as single action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
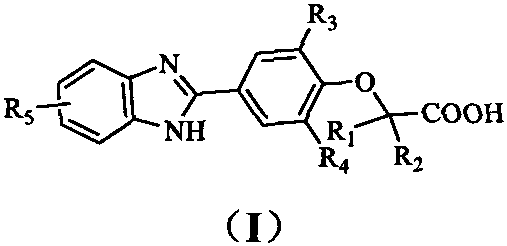
[0067] 2-Methyl-2-(2-methyl-4-(6-methyl-1H-benzo[d]imidazol-2-yl)phenoxy)propanoic acid (I-1)
[0068]
[0069] first step
[0070] Methyl 2-(4-formyl-2-methylphenoxy)-2-methylpropanoate (2a)
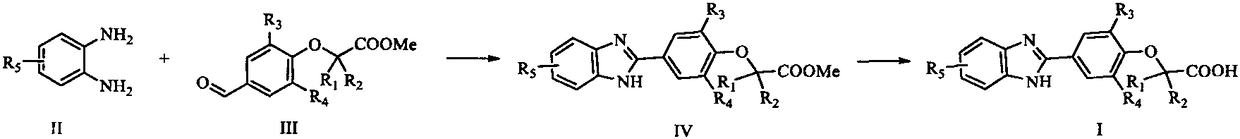
[0071] Dissolve 3-methyl-4-hydroxybenzaldehyde 1a (1.0g, 7.35mmol) and methyl 2-bromoisobutyrate (4.0g, 22.1mmol) in acetonitrile (20mL), add potassium carbonate (3.0g, 22.1mmol), reacted at 50°C for 12h, after TLC detected that the reaction was complete, added water to dissolve the solid mixture in the reaction, extracted with ethyl acetate (30mL×4), combined the organic phases with water (20mL×1), 1NHCl (20mL×1 ), washed with saturated NaCl solution (20mL×2), dried, concentrated, and the resulting product was directly used in the next reaction without purification.
[0072] second step
[0073] Methyl 2-methyl-2-(2-methyl-4-(6-methyl-1H-benzo[d]imidazol-2-yl)phenoxy)propanoate (3a)
[0074] Dissolve 2a (0.5g, 2.12mmol) and 3,4-diaminotoluene (0.29g, 2.12mmol) in DMF (15mL) with...
Embodiment 2
[0080] 2-Methyl-2-(2-methyl-4-(6-(trifluoromethyl)-1H-benzo[d]imidazol-2-yl)phenoxy)propanoic acid (I-2)
[0081]
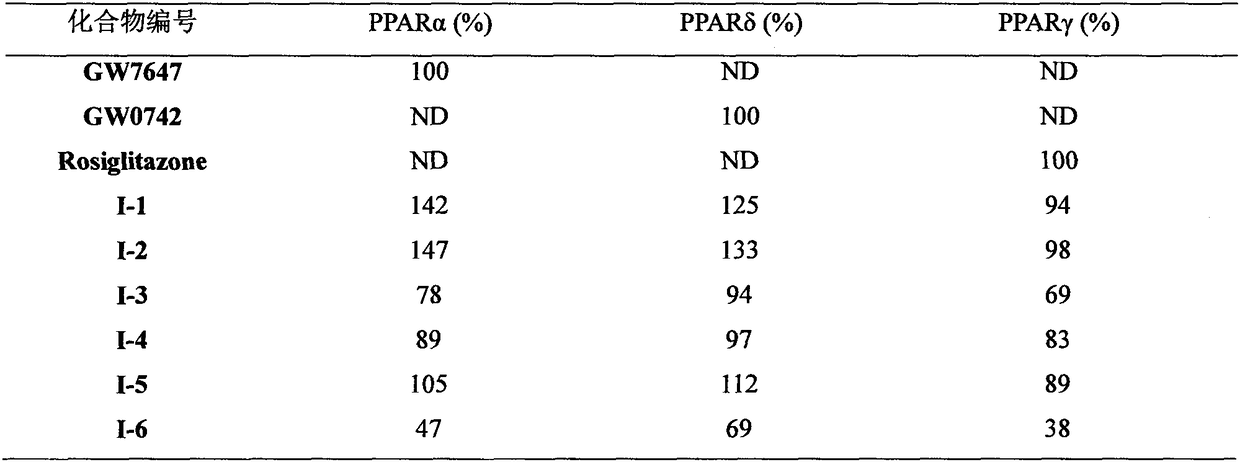
[0082] Referring to the preparation method of I-1, 1.5 g of white solid was obtained with a yield of 43%.
[0083] 1 H NMR (300MHz, D 2 O) δ: 10.67-10.44(m, 2H), 10.26(s, 1H), 10.17(d, J=8.5Hz, 1H), 10.00(d, J=8.5Hz, 1H), 9.09(d, J= 8.7Hz, 1H), 5.37(s, 1H), 4.47(s, 3H), 3.84(s, 6H). 13 C NMR (75MHz, DMSO-d 6 )δ: 174.87, 158.57, 151.79, 135.46, 132.90, 131.31, 129.53, 128.01, 126.47, 126.05, 125.62, 122.27, 115.79, 115.35, 111.67, 79.69, 25.60, 137.87 m.z [ES-MS] m.z - .Anal.calcd.For C 19 h 17 f 3 N 2 o 3 : C, 60.32; H, 4.53; N, 7.40; Found: C, 60.15; H, 4.38; N, 7.52.
Embodiment 3
[0085] 2-(2-fluoro-4-(6-methyl-1H-benzo[d]imidazol-2-yl)phenoxy)-2-methylpropanoic acid (I-3)
[0086]
[0087] Referring to the preparation method of I-1, 0.29 g of white solid was obtained with a yield of 49%.
[0088] 1 H NMR (300MHz, D 2 O) δ: 10.74-10.57(m, 1H), 10.44(d, J=8.7Hz, 1H), 9.89(d, J=8.4Hz, 1H), 9.80(s, 1H), 9.56(d, J= 8.4Hz, 1H), 9.38(t, J=8.6Hz, 1H), 5.37(s, 1H), 4.69(s, 3H), 3.85(s, 6H). 13 C NMR (75MHz, DMSO-d 6 )δ: 174.23, 147.62, 147.38, 136.27, 132.54, 130.41, 127.75, 125.41, 119.85, 116.52, 113.90, 113.69, 81.16, 25.43, 21.64. ESI-MS m / z: 327.1 [M-H] - .Anal.calcd.For C 18 h 17 FN 2 o 3 : C, 65.85; H, 5.22; N, 8.53; Found: C, 65.67; H, 5.35; N, 8.58.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com