Novel preparation method of nucleoside modified 5'-dmtr-2'-eoe-5-me-cytidine nucleoside
A nucleoside and compound technology, applied in the field of nucleoside compound synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
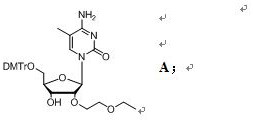
[0061] In a specific embodiment of the present invention, a preparation method of a compound of formula A is provided, comprising the steps of:
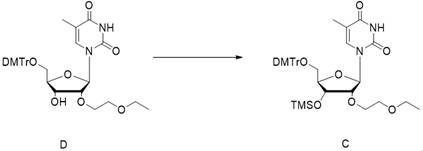
[0062] In the first step, the compound D was dissolved in the dichloromethane / triethylamine system, cooled to about 0°C, and then the R1-Cl reagent was added dropwise to obtain the crude compound C;
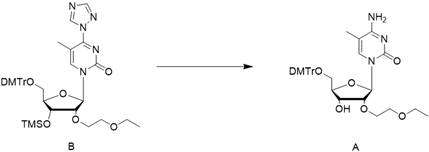
[0063] In the second step, dissolve the triazole in the dichloromethane / triethylamine system, lower the temperature to about 0°C, and then add the chlorination reagent dropwise; then add the reaction solution of compound C obtained in the first step into the reaction kettle dropwise , to obtain the crude compound B reaction solution;
[0064] In the third step, the crude compound B reaction solution was washed with water, concentrated to dryness, and the crude compound B was obtained;
[0065] In the fourth step, the crude compound B is dissolved in the ammonia water / acetonitrile system, and after stirring and reacting, the crude compo...
Embodiment 1
[0084] Embodiment 1, the preparation of compound C
[0085]
[0086] Weigh 47.70 kg of 5'-DMTr-2'-EOE-thymidine, add 500 L of dichloromethane (DCM) to dissolve, cool the reaction solution to 0°C, add 22.89 kg of triethylamine (TEA), stir for 30 min, and pour into the system 16.38 kg of TMS-Cl was added dropwise, the temperature of the system was kept at 0°C during the dropwise addition, and the reaction was stirred at 0°C after the dropwise addition until the raw materials were completely reacted to obtain the crude compound C. This reaction solution was directly used in the next step without treatment.
Embodiment 2
[0087] Embodiment 2, the preparation of compound B
[0088]
[0089] In another reaction kettle, add 480 L of dichloromethane (DCM), 31.24 kg of triazole, and 61.03 kg of triethylamine, and stir for 30 min. Cool in an ice bath to 0°C, add POCl dropwise to the system 3 17.34kg, stirring for 30 minutes after dropping. Then, at 0°C, the crude product of the reaction solution of compound C was added dropwise into the reaction kettle; after the dropwise addition, the reaction was stirred at 25°C until the reaction of raw material C was complete. Then the reaction solution was quenched with water, washed with water, and the organic phase was concentrated to dryness to obtain a crude compound B. This crude product was directly used in the next reaction without further purification.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com