High throughput screening method for monoclonal antibody product cell strains through two-dimensional liquid phase
A cell line, high-throughput technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of lengthy sample processing and storage steps, reduce the accuracy of analysis results, increase the probability of sample degradation, etc., to save manpower , improve efficiency and avoid degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
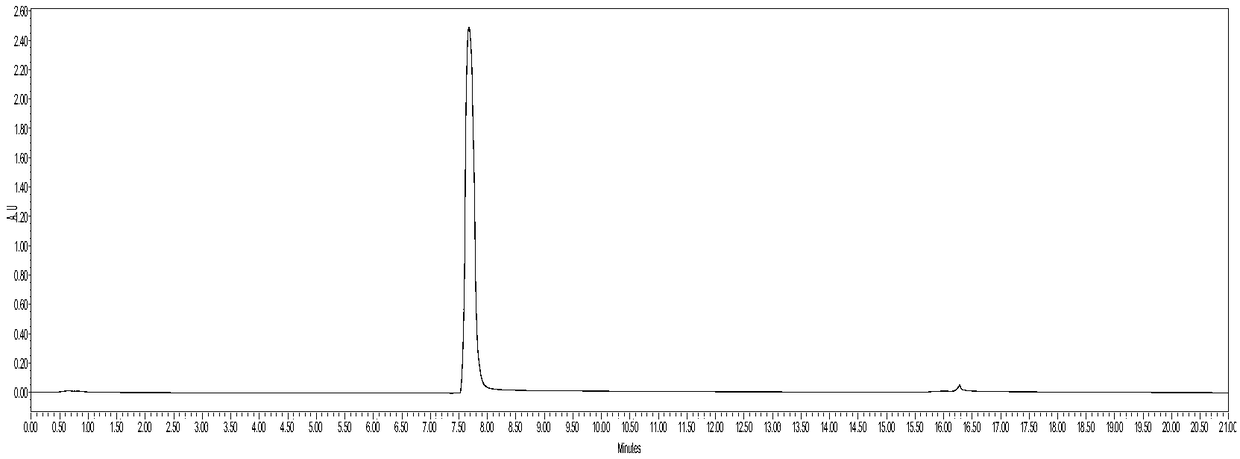
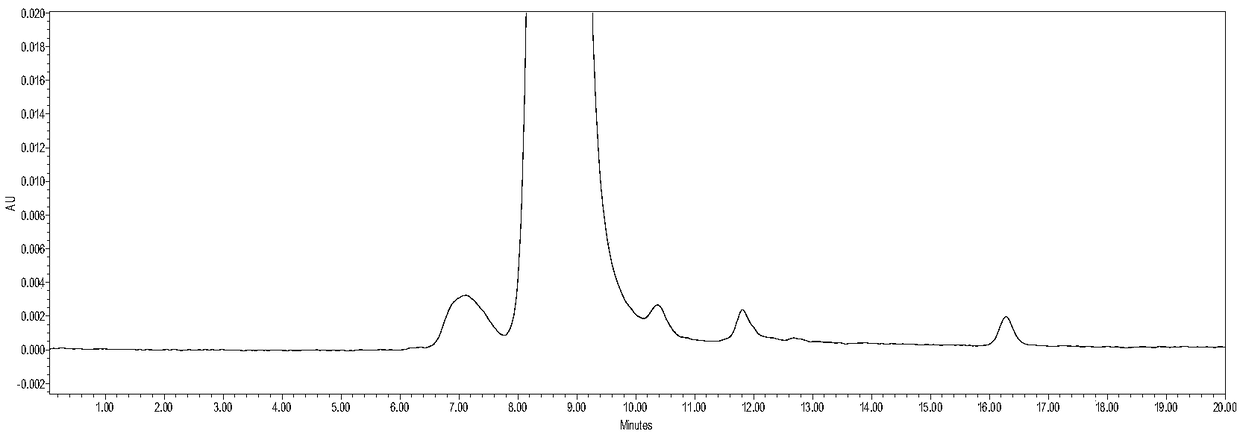
[0023] The cell line of bevacizumab was placed in SFM Ⅰ Ⅰ medium supplemented with 4 mmol / L glutamic acid, and the medium was filtered with a 0.2 μm filter membrane to remove cells and other larger particles to prepare the sample to be tested.
[0024] The detection of bevacizumab by two-dimensional liquid phase is used for high-throughput screening of bevacizumab cell lines. The conditions for separation by first-dimensional liquid chromatography are as follows: an affinity column is used to add 40 mmol The Tris-HCl buffer solution with a pH value of 6.3 as the mobile phase A, the buffer solution with a pH value of 2.5 as the mobile phase B, the flow rate is 0.3mL / min, the column temperature is 25°C, and the detection wavelength 280nm, gradient elution conditions: 0~3min, 0%B; 3~3.1min, 0%~100%B; 3.1~6min, 100%B; 6~6.1min, 100%~0%B; 6.1 ~10min, 0% B; the condition that the second-dimension liquid chromatography carries out detection is: adopt size-exclusion chromatographic co...
Embodiment 2
[0031] The cell line of bevacizumab was placed in SFM Ⅰ Ⅰ medium supplemented with 4 mmol / L glutamic acid, and the medium was filtered with a 0.2 μm filter membrane to remove cells and other larger particles to prepare the sample to be tested.
[0032] Bevacizumab is detected by two-dimensional liquid phase to perform high-throughput screening of bevacizumab cell lines. The conditions for separation by first-dimensional liquid chromatography are: use an affinity chromatographic column to add 60 mmol The Tris-HCl buffer solution whose pH value is 7.3 of the NaCl eluent is mobile phase A, and the buffer solution with a pH value of 3.5 is mobile phase B. The flow rate is 0.5mL / min, the detection wavelength is 280nm, and the column temperature is 25°C, gradient elution conditions: 0~3min, 0%B; 3~3.1min, 0%~100%B; 3.1~6min, 100%B; 6~6.1min, 100%~0%B; 6.1 ~10min, 0% B; the condition that the second-dimension liquid chromatography carries out detection is: adopt size-exclusion chroma...
Embodiment 3
[0039] The cell line of bevacizumab was placed in SFM Ⅰ Ⅰ medium supplemented with 4 mmol / L glutamic acid, and the medium was filtered with a 0.2 μm filter membrane to remove cells and other larger particles to prepare the sample to be tested.
[0040]The detection of bevacizumab by two-dimensional liquid phase is used for high-throughput screening of bevacizumab cell lines. The conditions for separation by first-dimensional liquid chromatography are as follows: an affinity column is used to add 50 mmol The Tris-HCl buffer solution with a pH value of 6.8 as the mobile phase A, the buffer solution with a pH value of 3.0 as the mobile phase B, the flow rate is 0.5mL / min, the detection wavelength is 280nm, and the column temperature is 25°C, gradient elution conditions: 0~3min, 0%B; 3~3.1min, 0%~100%B; 3.1~6min, 100%B; 6~6.1min, 100%~0%B; 6.1 ~10min, 0% B; the condition that the second-dimension liquid chromatography carries out detection is: adopt size-exclusion chromatographic ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com