Health-care wine prepared from desert cistanche and Korean pine seeds and preparation method thereof
A technology of Cistanche deserticola and sea pine nuts, which is applied in the field of health wine and tablets, can solve the problems affecting the modernization and internationalization process, the decline of the comprehensive curative effect of drugs, and the unstable product quality, so as to solve the problem of effective substance transfer rate and production efficiency, clinical The effect of definite curative effect and clear active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
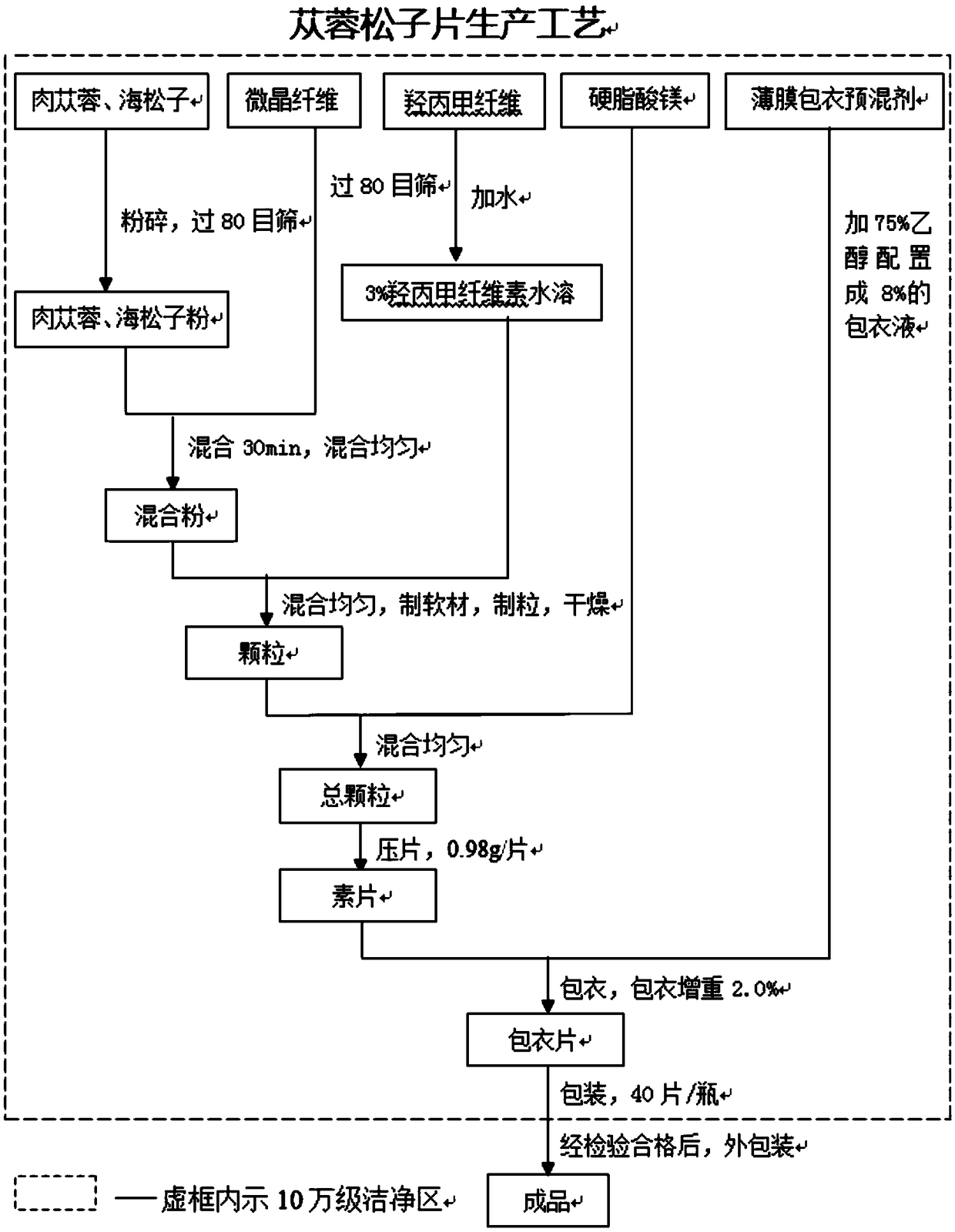
Image
Examples
Embodiment 1
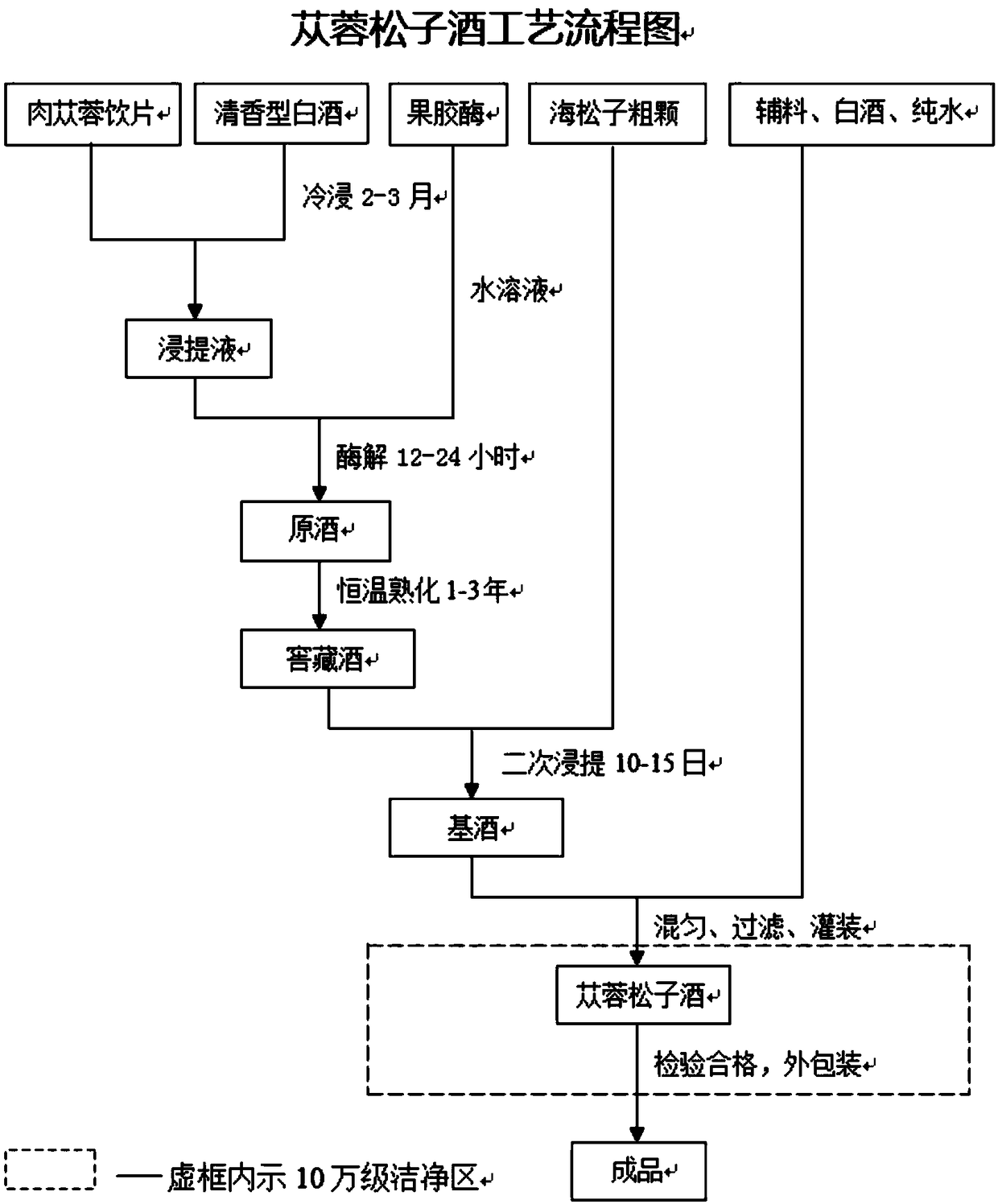
[0059] 1, a kind of prescription and preparation technology of desert cistanche pine wine, cistanche pine wine is characterized in that, formula is cistanche 15g-90g, sea pine nut 10-50g. Appropriate amount of rock sugar, red yeast rice, and honey are used as supplementary materials, and 55-60±1 (v / v) fragrant liquor is used as the base, and the 38- 52±1 (v / v) liquor 1000ml.
[0060] Specific steps are as follows:
[0061] (1) Clean selection of raw materials: dry decoction pieces of Cistanche deserticola, 60 g of which are cleaned to remove impurities; sea pine nuts are dried seeds, seed coats are removed, and the seed kernels are coarsely powdered into granules (less than 1.2 mm) after cleaning, and 35 g of them are used for future use.
[0062] (2) Feeding: Weigh Cistanche raw materials and auxiliary materials according to the composition and ratio of the formula, put the Cistanche decoction pieces and rock sugar coarse particles into the extraction tank (with agitator), a...
experiment example 2
[0078] Experimental example 2: Animal experiments
[0079] 1 Experimental materials
[0080] 1.1 Drugs
[0081] 1.1.1 Drug of the present invention: Prepare according to Example 1.
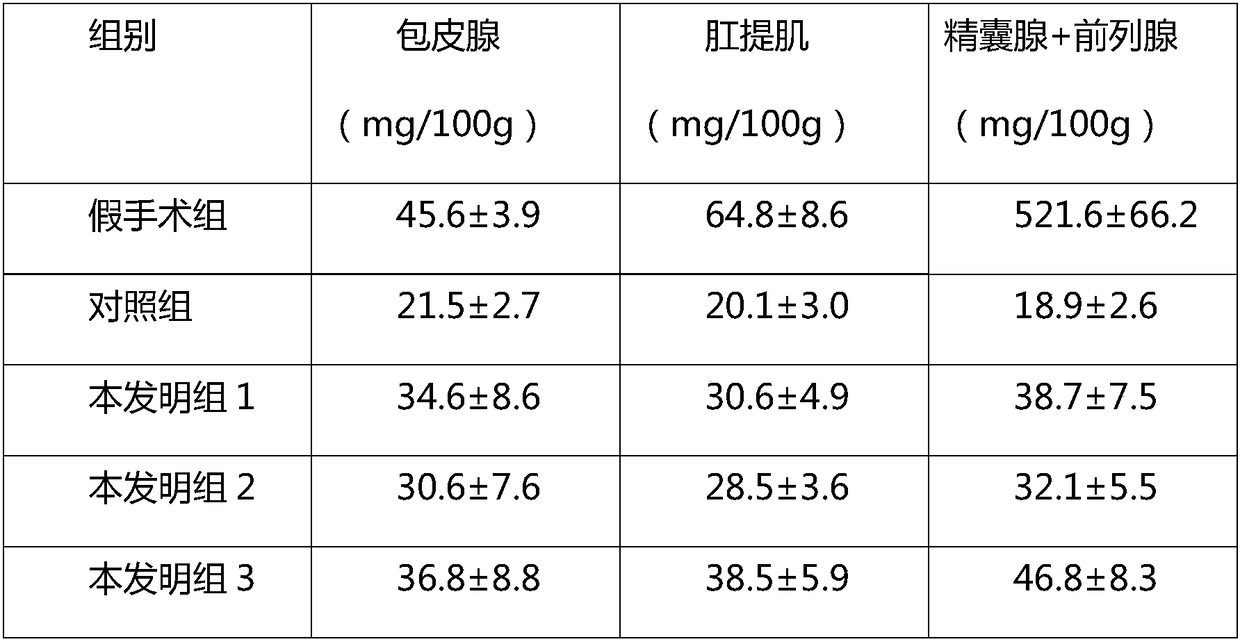
[0082] 1.2 Experimental animals: male rats, weighing 80-100g, 50 rats, clean grade.
[0083] 2 methods
[0084] Take 50 male rats weaned for 1 month, weighing 80-100g, after subcutaneous anesthesia, the dorsal position is fixed, and 40 of the animals have their bilateral testes surgically removed; the other 10 rats are subjected to sham surgery, that is, only the scrotum is cut open The skin is removed without removing the testicles, and the wound is then sutured. After the operation, each rat was subcutaneously injected with penicillin sodium 20,000 U / rat for 3 consecutive days.
[0085] Castrated rats are randomly divided into (1) sham-operated control group (distilled water 0.05ml / 100g) according to body weight; (2) matched group (distilled water 0.05ml / 100g); (3) the present invention gro...
Embodiment 3
[0091] Embodiment 3, clinical experiment
[0092] A total of 60 cases were collected, drinking 1-1.5 taels of health wine every day at lunch, and taking 1-2 tablets before going to bed. The course of treatment was 30 days, of which 2 cases were cured (3.3%), and 16 cases (26.7%) were markedly effective , progressed in 34 cases (56.7%), 8 cases (13.3%) were ineffective, and the total effective rate was 86.7%. The results show that the product of the invention can effectively alleviate the symptoms and signs of patients with kidney deficiency.
[0093] Recovery: clinical symptoms and signs of kidney deficiency disappear
[0094] Significantly effective: the clinical symptoms and signs of kidney deficiency are significantly improved
[0095] Progress: clinical symptoms and signs of kidney deficiency have improved
[0096] Ineffective: The clinical symptoms and signs of kidney deficiency have no obvious improvement or aggravation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com