Stable compound preparation valsartan amlodipine recipe and preparation method thereof
A technology of valsartan amlodipine tablets and amlodipine besylate, which is applied in the field of preparation of valsartan amlodipine tablets, can solve problems such as high temperature instability of raw materials, achieve improved bioavailability, and solve content problems, the effect of improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
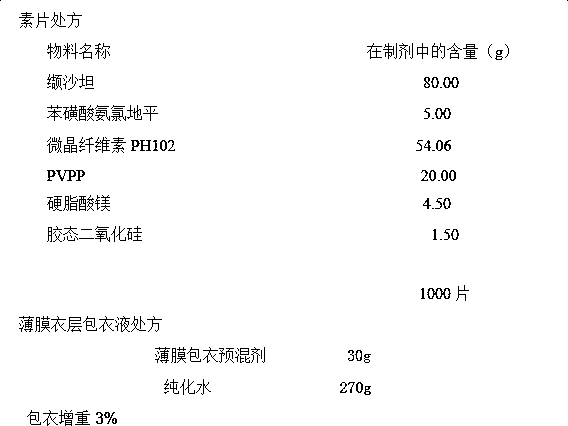
[0024] plain tablet prescription
[0025]
[0026] Preparation:
[0027] Weigh the prescribed amount of amlodipine besylate and microcrystalline cellulose PH102, and premix them in an equal-volume incremental mixing method. Weigh the prescribed amount of other raw materials and all excipients, and mix for about 3 minutes.
[0028] Fill the mixed material into the dry granulator, set the tableting pressure to 2.5MPa, the feeding frequency to 10.0Hz, the tableting frequency to 10.0Hz, and the granulation frequency to 10.0Hz, use a 30-mesh sieve for granulation, add The additional materials in the prescribed amount are mixed evenly and compressed with a tablet machine.
[0029] Coating in a coating pan with 10% coating solution, and increasing the weight by 3%, to obtain a finished product.
Embodiment 2
[0031]
[0032] Preparation:
[0033] Weigh the prescribed amount of amlodipine besylate and microcrystalline cellulose PH102, and premix them in an equal-volume incremental mixing method. Weigh the prescribed amount of other raw materials and all excipients, and mix for about 3 minutes.
[0034] Fill the mixed material into a wet granulator, add 10% sodium carboxymethyl cellulose aqueous solution to make a soft material, granulate with a 24-mesh sieve, dry, granulate with a 30-mesh sieve, and compress with a tablet machine.
[0035] Coating in a coating pan with 10% coating solution, and increasing the weight by 3%, to obtain a finished product.
Embodiment 3
[0037]
[0038] Preparation:
[0039] Weigh the prescribed amount of amlodipine besylate and microcrystalline cellulose PH102, and premix them in an equal-volume incremental mixing method. Weigh the prescribed amount of other raw materials and all excipients, and mix for about 3 minutes.
[0040] Fill the mixed material into the dry granulator, set the tableting pressure to 2.5MPa, the feeding frequency to 10.0Hz, the tableting frequency to 10.0Hz, and the granulation frequency to 10.0Hz, use a 30-mesh sieve for granulation, and Tablet press tablet.
[0041] Coating in a coating pan with 10% coating solution, and increasing the weight by 3%, to obtain a finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com