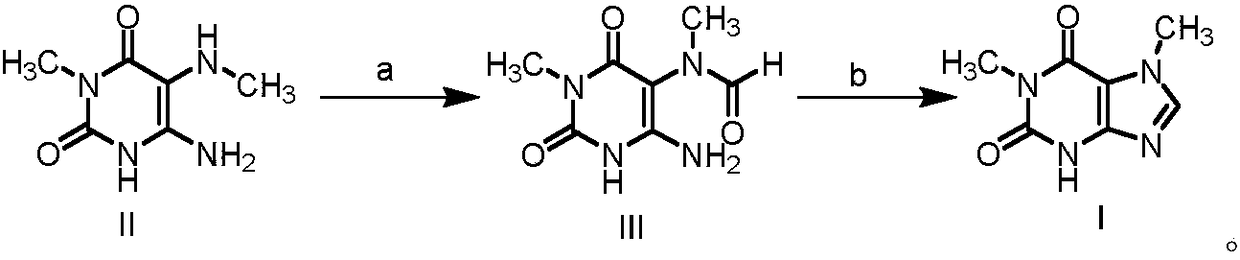
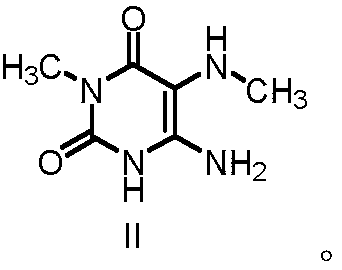
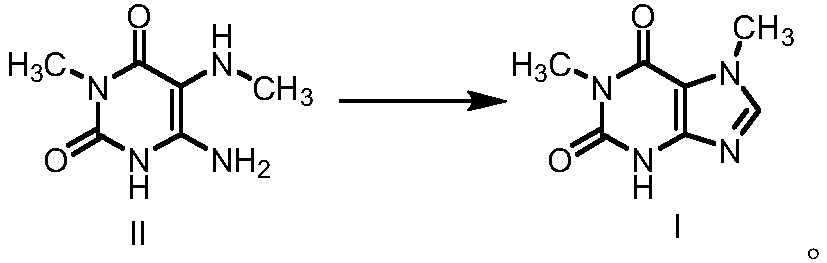Preparation methods of 1, 7-dimethylxanthine and intermediate thereof, and intermediate
A technology of dimethylxanthine and dimethylformamide, which is applied in the field of medicinal chemistry, can solve the problems of harsh conditions, inability to scale up 7-methyladenine benzylium salt derivatives, and low debenzylation yield. Achieving mild reaction conditions, avoiding column chromatography, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Preparation of 6-amino-3-methyl-5-(methylamino)pyrimidinyl-2,4(1H,3H)-dione
[0066]
[0067] 6-Amino-3-methylpyrimidinyl-2,4(1H,3H)-dione (10g, 70.9mmol) was dissolved in acetonitrile, and N-chlorosuccinimide ( 12.9g, 96.8mmol), and react at 20-30°C for 6 hours after the addition, and the reaction is basically complete. After concentration, add water and dichloromethane, separate the liquids, wash with sodium thiosulfate solution, sodium bicarbonate solution, and water successively, concentrate the organic phase and recrystallize, and dry to obtain a light yellow solid 6-amino-5-chloro-3- 10.4 g (57.0 mmol) of methylpyrimidinyl-2,4(1H,3H)-dione, the yield is 80%.
[0068] 6-Amino-5-chloro-3-methylpyrimidinyl-2,4(1H,3H)-dione (10.4g, 57.0mmol) was suspended in ethanol, and 30% methylamine ethanol solution (28.3g , 274mmol), reacted at 20-30°C for 12 hours, and the reaction was almost complete. Concentrate, add dichloromethane, water solution, concentrate...
Embodiment 2
[0069] Example 2 Preparation of 6-amino-3-methyl-5-(methylamino)pyrimidinyl-2,4(1H,3H)-dione
[0070]
[0071] 6-Amino-3-methylpyrimidinyl-2,4(1H,3H)-dione (10g, 70.9mmol) was dissolved in acetonitrile, and dibromohydantoin (13.8g, 48.4mmol ). After the addition is complete, react at 20-30°C for 6 hours, and the reaction is basically complete. After concentration, add water and dichloromethane, separate the liquids, wash with sodium thiosulfate solution, sodium bicarbonate solution, and water successively, concentrate the organic phase and recrystallize, and dry to obtain a light yellow solid 6-amino-5-bromo-3- Methylpyrimidinyl-2,4(1H,3H)-dione 12.9g (58.6mmol), yield 83%.
[0072] 6-Amino-5-bromo-3-methylpyrimidinyl-2,4(1H,3H)-dione (12.9g, 58.6mmol) was suspended in methyl tetrahydrofuran, and a 30% methanol solution of methylamine was added ( 17.1g, 165mmol), reacted at 20-30°C for 12 hours, and the reaction was almost complete. Concentrate, add dichloromethane and ...
Embodiment 3
[0073] Example 3 Preparation of 6-amino-3-methyl-5-(methylamino)pyrimidinyl-2,4(1H,3H)-dione
[0074]
[0075]6-Amino-3-methylpyrimidinyl-2,4(1H,3H)-dione (10g, 70.9mmol) was dissolved in acetonitrile, and dibromohydantoin (30.3g, 106.4mmol ). After the addition is complete, react at 20-30°C for 6 hours, and the reaction is basically complete. After concentration, add water and dichloromethane, separate the liquids, wash with sodium thiosulfate solution, sodium bicarbonate solution, and water successively, concentrate the organic phase and recrystallize, and dry to obtain a light yellow solid 6-amino-5-bromo-3- Methylpyrimidinyl-2,4(1H,3H)-dione 13.7g (62.4mmol), yield 88%.
[0076] 6-Amino-5-bromo-3-methylpyrimidinyl-2,4(1H,3H)-dione (12.9g, 58.6mmol) was suspended in methyl tetrahydrofuran, and a 30% concentration of methylamine solution (1172mmol ), reacted at 20-30° C. for 12 hours, and the reaction was basically complete. Concentrate, add dichloromethane and water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com