Medicine composition for treatment of diabetes, and preparation method and application thereof
A composition and diabetes technology, applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., can solve the problems of prevention or delay of diabetes complications without evidence-based medicine, renal function damage, renal disease aggravation, etc., to achieve Control blood sugar production rate, increase compliance, good synergistic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
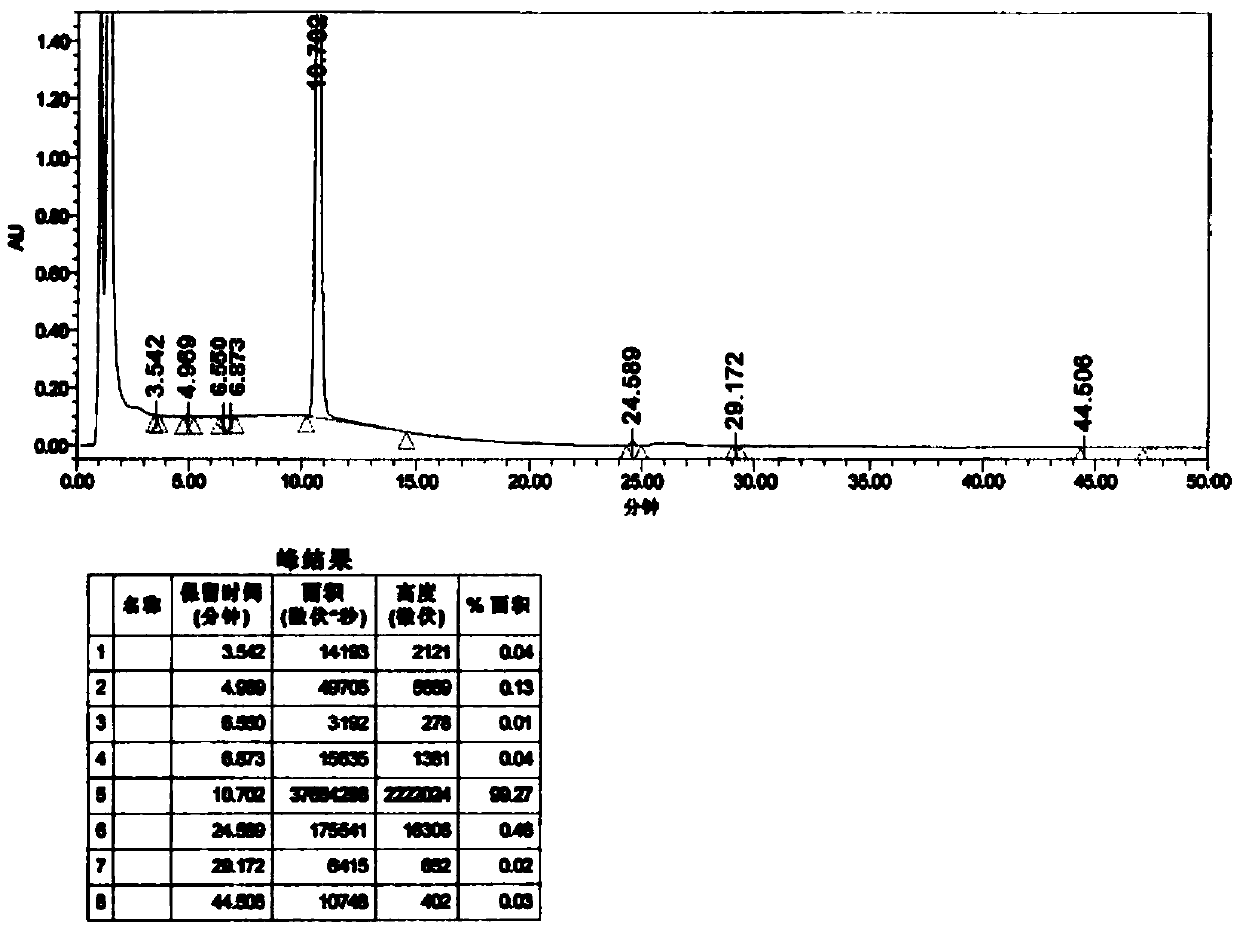
[0077] This example is used to illustrate the pharmaceutical composition of tepagliflozin and metformin hydrochloride of the present invention and its preparation method, and the specific details are as follows: specification 2.5mg / 250mg
[0078]
[0079] Preparation Process:
[0080] Pass the prescribed amount of metformin hydrochloride and polyvinylpyrrolidone through an 80-mesh sieve, and then put them into the silo of the wet granulator, and dry-mix at a speed (stirring 450rpm, shearing 470rpm) for 5-10 minutes. Add an appropriate amount of 50% ethanol water, and wet the granules at a speed (stirring 450 rpm, shearing 470 rpm) for 3 to 5 minutes. The wet granules are granulated through a 20-mesh sieve with a swing granulator. After the granulation, the wet granules are poured into the Glatt fluidized bed machine, the air inlet temperature is 50-60°C, the material temperature is about 50°C, and the drying time is about 8-14 minutes. Control moisture ≤ 3.5% is the dryin...
Embodiment 2
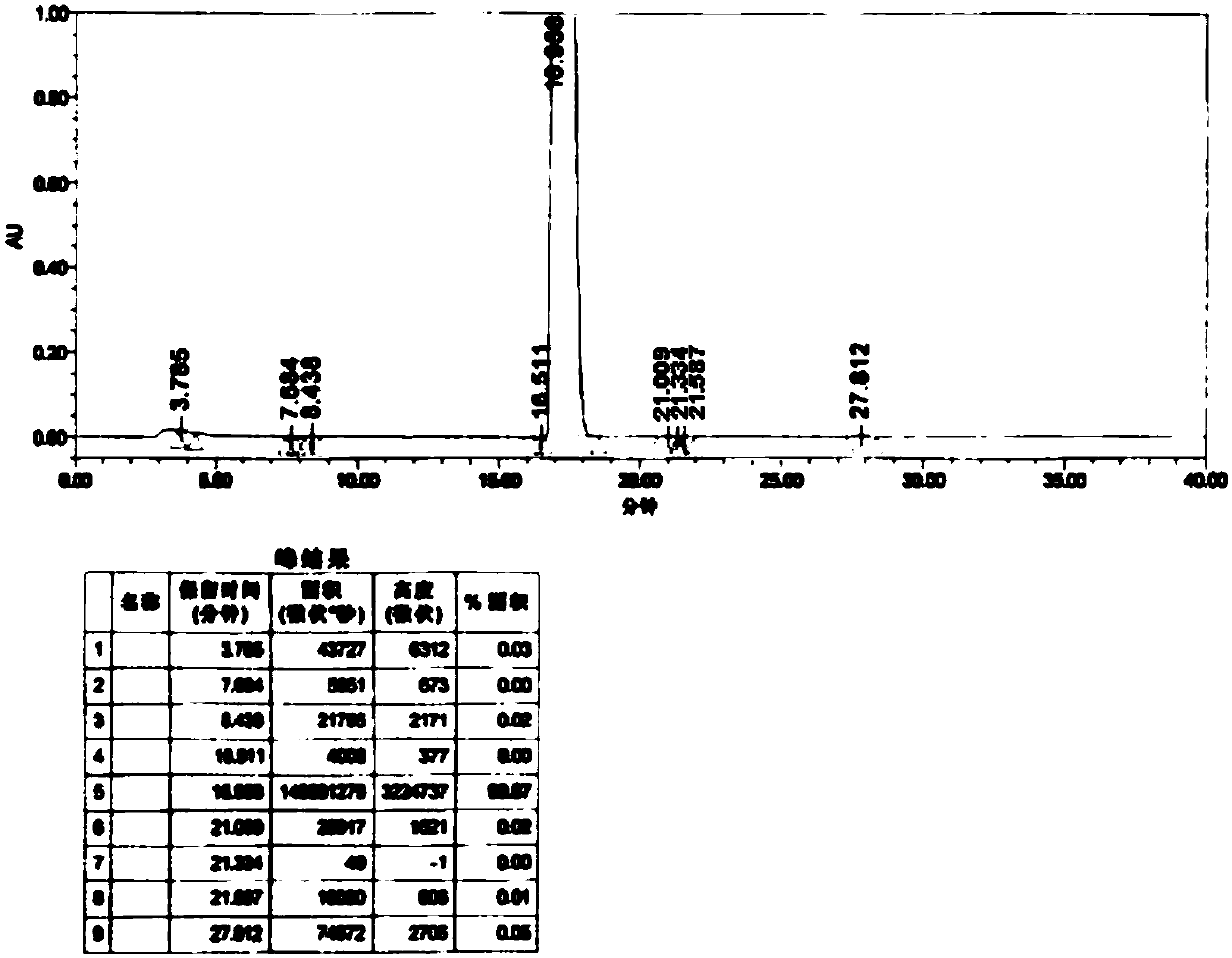
[0086] This example is used to illustrate the pharmaceutical composition of tepagliflozin and metformin hydrochloride of the present invention and its preparation method, and the specific details are as follows: specification 5mg / 250mg
[0087]
[0088] Preparation Process:
[0089] Pass the prescribed amount of metformin hydrochloride and hypromellose through an 80-mesh sieve, then put them into the silo of the wet granulator, and dry mix at a speed (stirring 450rpm, shearing 470rpm) for 5-10 minutes. Add an appropriate amount of 30% ethanol water, and wet the granules at a speed (stirring 450 rpm, shearing 470 rpm) for 3 to 5 minutes. The wet granules are granulated through a 20-mesh sieve with a swing granulator. After the granulation, the wet granules are poured into the Glatt fluidized bed machine, the air inlet temperature is 50-60°C, the material temperature is about 50°C, and the drying time is about 8-14 minutes. Control moisture ≤ 3.5% is the drying end point. Th...
Embodiment 3
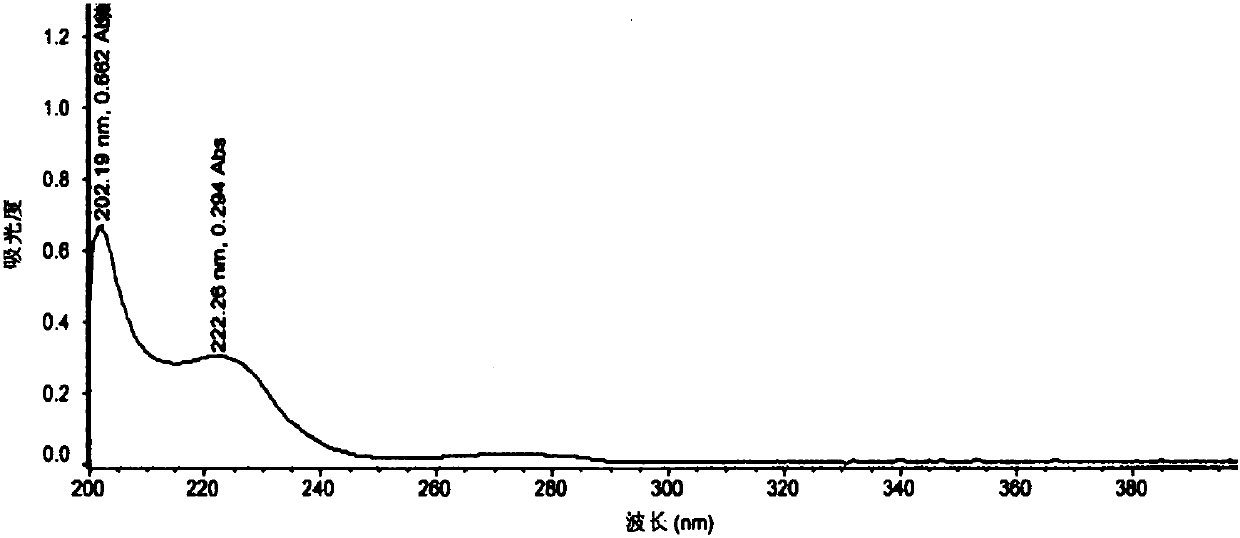
[0095] This example is used to illustrate the pharmaceutical composition of tepagliflozin and metformin hydrochloride of the present invention and its preparation method, specifically detailed as follows: specification 5mg / 500mg
[0096]
[0097] Preparation Process:
[0098] Pass the prescribed amount of metformin hydrochloride and polyvinylpyrrolidone through an 80-mesh sieve, and then put them into the silo of the wet granulator, and dry-mix at a speed (stirring 480rpm, shearing 500rpm) for 5-10 minutes. Add an appropriate amount of 50% ethanol water, and wet the granules at a speed (stirring 480 rpm, shearing 500 rpm) for 3 to 5 minutes. The wet granules are granulated through a 20-mesh sieve with a swing granulator. After the granulation, the wet granules are poured into the Glatt fluidized bed machine, the air inlet temperature is 50-60°C, the material temperature is about 50°C, and the drying time is about 8-14 minutes. Control moisture ≤ 3.5% is the drying end poi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com