CO2 low-temperature methanation catalyst, preparation and application thereof
A low-temperature methanation and catalyst technology, used in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, and hydrocarbon production from carbon oxides, etc., to achieve high low-temperature activity, good catalyst stability, and preparation. simple method effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
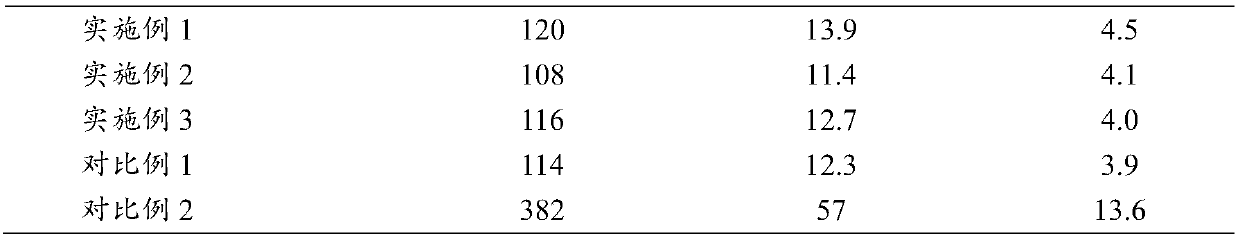
Embodiment 1
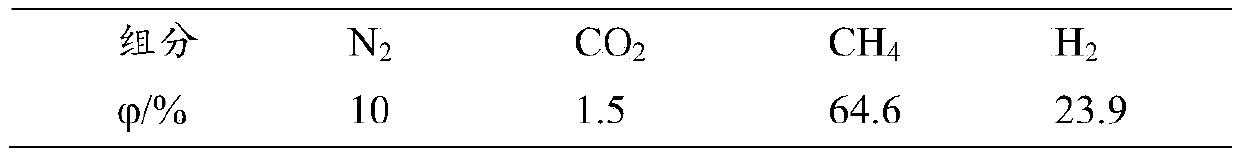
[0027] Weigh 43.27g of aluminum nitrate nonahydrate and dissolve it in 1000mL of deionized water, heat it to 50-70°C in a constant temperature reactor, add 82.35g of Al 2 o 3 and 11.76g of CNTs powder, stirred and slowly added dropwise 0.05g / mL potassium carbonate solution for precipitation reaction, the pH value at the end point was controlled to be 7.0-8.5, and the reaction time was about 30-40min. The precipitate was aged for 2 hours, washed, filtered, dried at 110°C for 8 hours, and then baked in a muffle furnace at 400°C for 4 hours to obtain carrier powder.
[0028] Crush the carrier powder through a 200-mesh sieve, add water and mix evenly, press it into a column shape with a ring press machine at Φ6×6mm, and dry at 110°C to obtain Al 2 o 3 - CNTs carrier.
[0029] Add the above carrier to excess 1.68g / mL Ni(NO 3 ) 2 ·6H 2 O and 0.18g / mLLa(NO 3 ) 3 ·6H 2 Immerse in the mixed solution of O for 0.5h, take out the solid after impregnation, dry in an oven at 100°C ...
Embodiment 2
[0032] Weigh 43.27g of aluminum nitrate nonahydrate, dissolve it in 1000mL of deionized water, heat it to 50-70°C in a constant temperature reactor, and add 76.47g of Al 2 o 3 and 17.65g of CNTs powder, stirred and slowly added dropwise 0.05g / mL potassium carbonate solution for precipitation reaction, the pH value at the end point was controlled to be 7.0-8.5, and the reaction time was about 30-40min. The precipitate was aged for 2 hours, washed, filtered, dried at 100° C. for 8 hours, and then placed in a muffle furnace for 350° C. and calcined for 4 hours to obtain carrier powder.
[0033] Crush the carrier powder through a 200-mesh sieve, add water and mix evenly, press it into a column shape with a ring press machine at Φ6×6mm, and dry at 110°C to obtain Al 2 o 3 - CNTs carrier.
[0034] Add the above carrier to excess 1.68g / mL Ni(NO 3 ) 2 ·6H 2 O and 0.18g / mLLa(NO 3 ) 3 ·6H 2 Immerse in the mixed solution of O for 0.5h, take out the solid after impregnation, dry ...
Embodiment 3
[0037] Weigh 43.27g of aluminum nitrate nonahydrate and dissolve it in 1000mL of deionized water, heat it to 50-70°C in a constant temperature reactor, add 82.35g of Al 2 o 3and 11.76g of CNTs powder, stirred and slowly added dropwise 0.05g / mL potassium carbonate solution for precipitation reaction, the pH value at the end point was controlled to be 7.0-8.5, and the reaction time was about 30-40min. The precipitate was aged for 2 hours, washed, filtered, dried at 110°C for 8 hours, and then baked in a muffle furnace at 400°C for 4 hours to obtain carrier powder.
[0038] Crush the carrier powder through a 200-mesh sieve, add water and mix evenly, press it into a column shape with a ring press machine at Φ6×6mm, and dry at 110°C to obtain Al 2 o 3 - CNTs carrier.
[0039] Add the above carrier to excess 1.68g / mL Ni(NO 3 ) 2 ·6H 2 O and 0.17g / mLCe(NO 3 ) 4 ˙6H 2 Immerse in a mixed solution of O for 0.5h, take out the solid after immersion, dry in an oven at 110°C for 8h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com