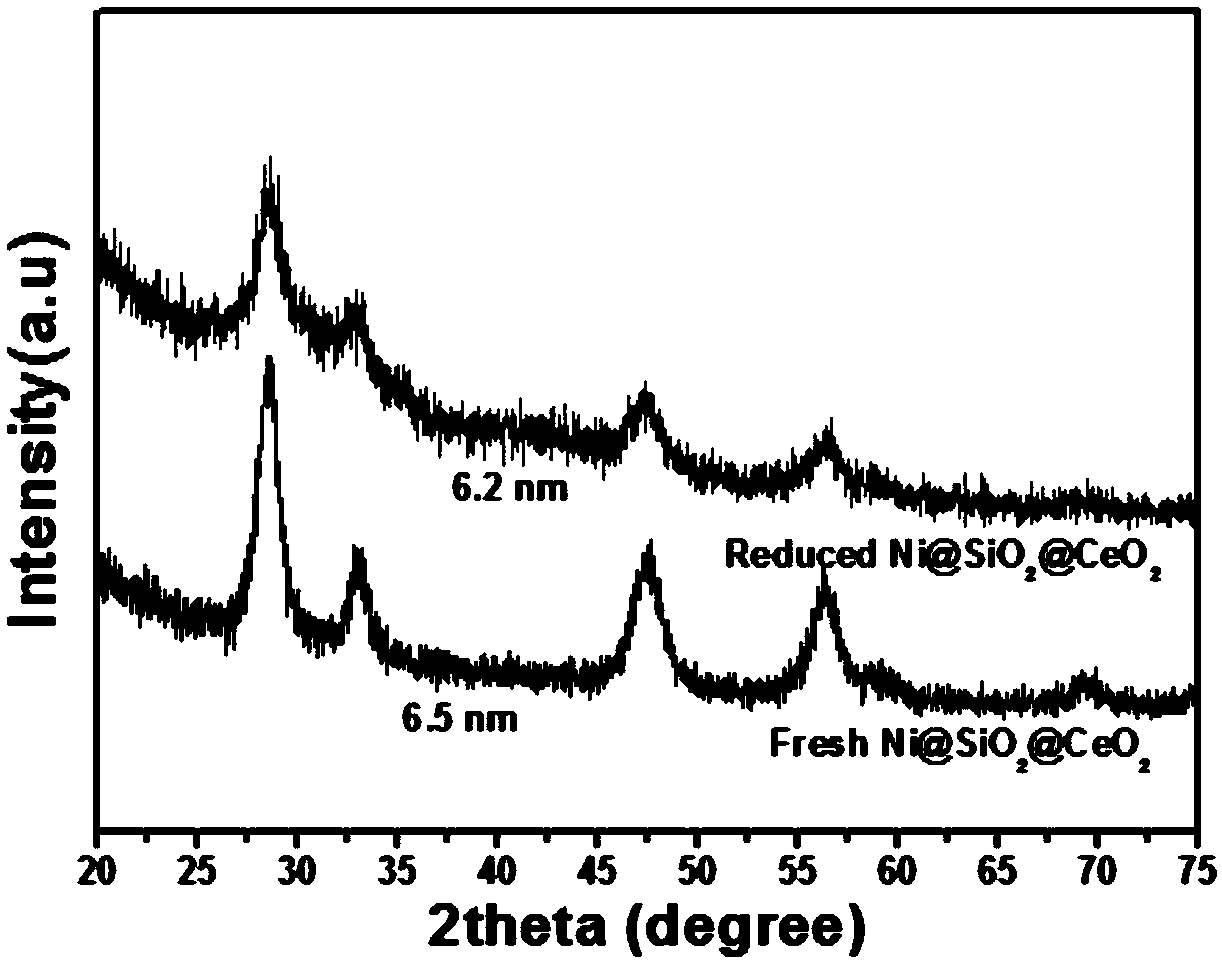
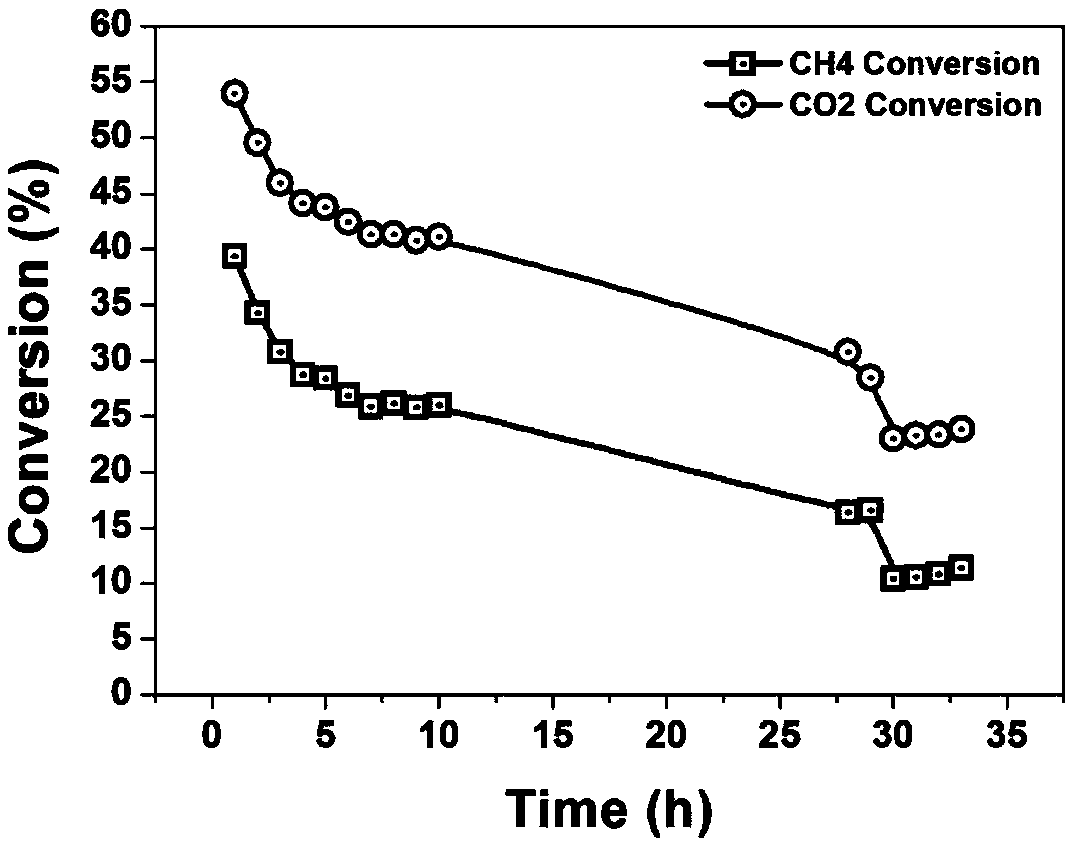
Preparation method of Ni@SiO2@CeO2 core-shell nanosphere catalyst and application thereof in methane carbon dioxide reforming reaction
A technology of core-shell catalysts and surfactants, which is applied in the fields of energy utilization and the environment, can solve the problems of reduced catalyst activity and no reports on low-temperature catalyst performance, and achieve high conversion rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] a. Mix 5.62 grams of polyoxyethylene alkyl ethers (n=20) and 15 ml of cyclohexane at room temperature to form a white emulsion, and add 0.5 ml of Ni(NO 3 ) 3 ·6H 2 O (1mol / L), stirred at room temperature for 30 minutes to form a light green emulsion.
[0034] b. Put the light green milk in a in a water bath at 50°C and stir for 5-10 minutes to form a light green solution. Slowly add 0.6ml of hydrazine hydrate dropwise to the light green solution and stir for 20 minutes to form a purple solution.
[0035] c. Slowly add 2.1ml of tetraethyl orthosilicate dropwise to the purple solution in c, stir for 10 minutes while adding dropwise, and slowly add 1.5ml of ammonia water for hydrolysis for 1 hour.
[0036] d. Then add isopropanol to terminate the reaction, wash the sample four times at a centrifugal speed of 8000 rpm with isopropanol, then place it in a 60°C oven and dry it for 12 hours to obtain [Ni(N 2 h 4 ) 3 ](NO 3 ) 2 @SiO 2 Precursor. [Ni(N 2 h 4 ) 3 ](NO...
Embodiment 2
[0052] a. Mix 5.62 grams of polyoxyethylene alkyl ethers (n=20) and 15 ml of cyclohexane at room temperature to form a white emulsion, and add 0.5 ml of Ni(NO 3 ) 3 ·6H 2 O (0.3mol / L), stirred at room temperature for 30 minutes to form a light green emulsion.
[0053] b. Put the light green milk in a in a water bath at 50°C and stir for 5-10 minutes to form a light green solution. Slowly add 0.6ml of hydrazine hydrate dropwise to the light green solution and stir for 20 minutes to form a purple solution.
[0054] c. Slowly add 2.1ml of tetraethyl orthosilicate dropwise to the purple solution in c, stir for 10 minutes while adding dropwise, and slowly add 1.5ml of ammonia water for hydrolysis for 1 hour.
[0055] d. Then add isopropanol to terminate the reaction, wash the sample four times at a centrifugal speed of 8000 rpm with isopropanol, then place it in a 60°C oven and dry it for 12 hours to obtain [Ni(N 2 h 4 ) 3 ](NO 3 ) 2 @SiO 2 Precursor. [Ni(N 2 h 4 ) 3 ](...
Embodiment 3
[0062] a. Mix 5.62 grams of polyoxyethylene alkyl ethers (n=20) and 15 ml of cyclohexane at room temperature to form a white emulsion, and add 0.5 ml of Ni(NO 3 ) 3 ·6H 2 O (1mol / L), stirred at room temperature for 30 minutes to form a light green emulsion.
[0063] b. Put the light green milk in a in a water bath at 50°C and stir for 5-10 minutes to form a light green solution. Slowly add 0.6ml of hydrazine hydrate dropwise to the light green solution and stir for 20 minutes to form a purple solution.
[0064] c. Slowly add 2.1ml of tetraethyl orthosilicate dropwise to the purple solution in c, stir for 10 minutes while adding dropwise, and slowly add 1.5ml of ammonia water for hydrolysis for 1 hour.
[0065] d. Then add isopropanol to terminate the reaction, wash the sample four times at a centrifugal speed of 8000 rpm with isopropanol, then place it in a 60°C oven and dry it for 12 hours to obtain [Ni(N 2 h 4 ) 3 ](NO 3 ) 2 @SiO 2 Precursor. [Ni(N 2 h 4 ) 3 ](NO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com