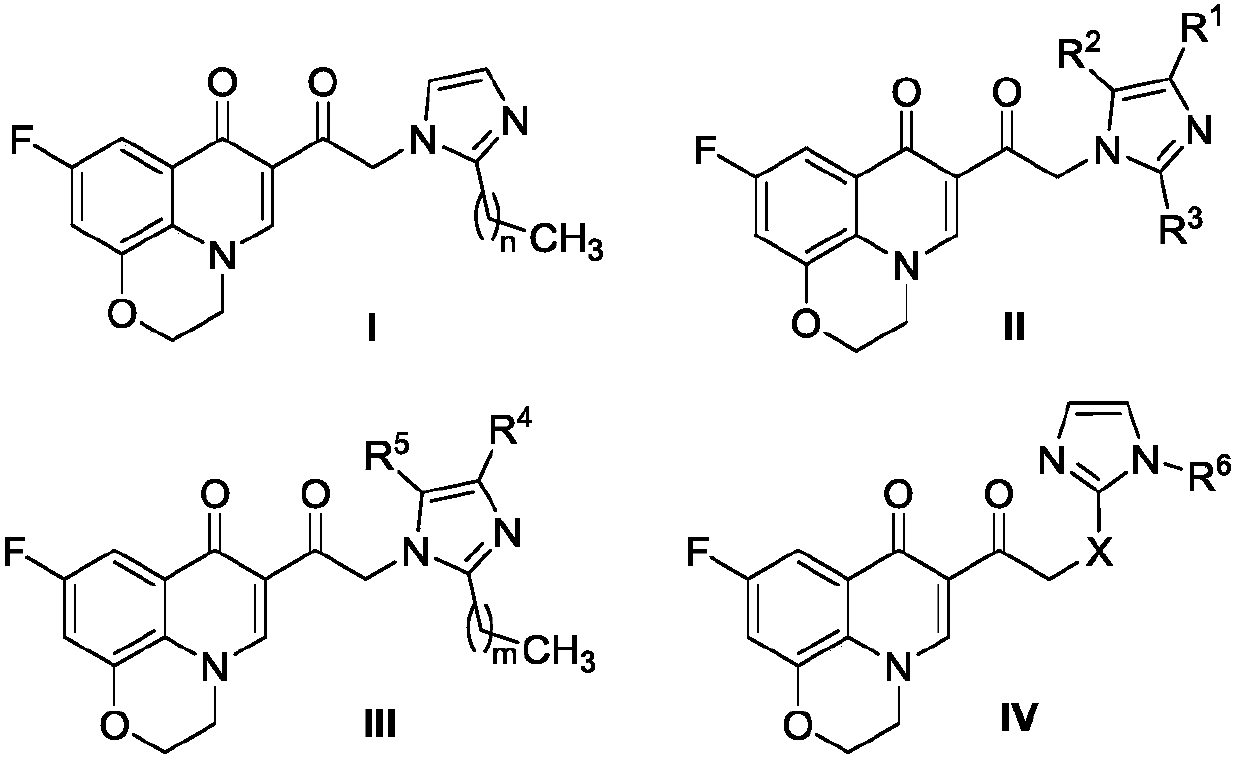
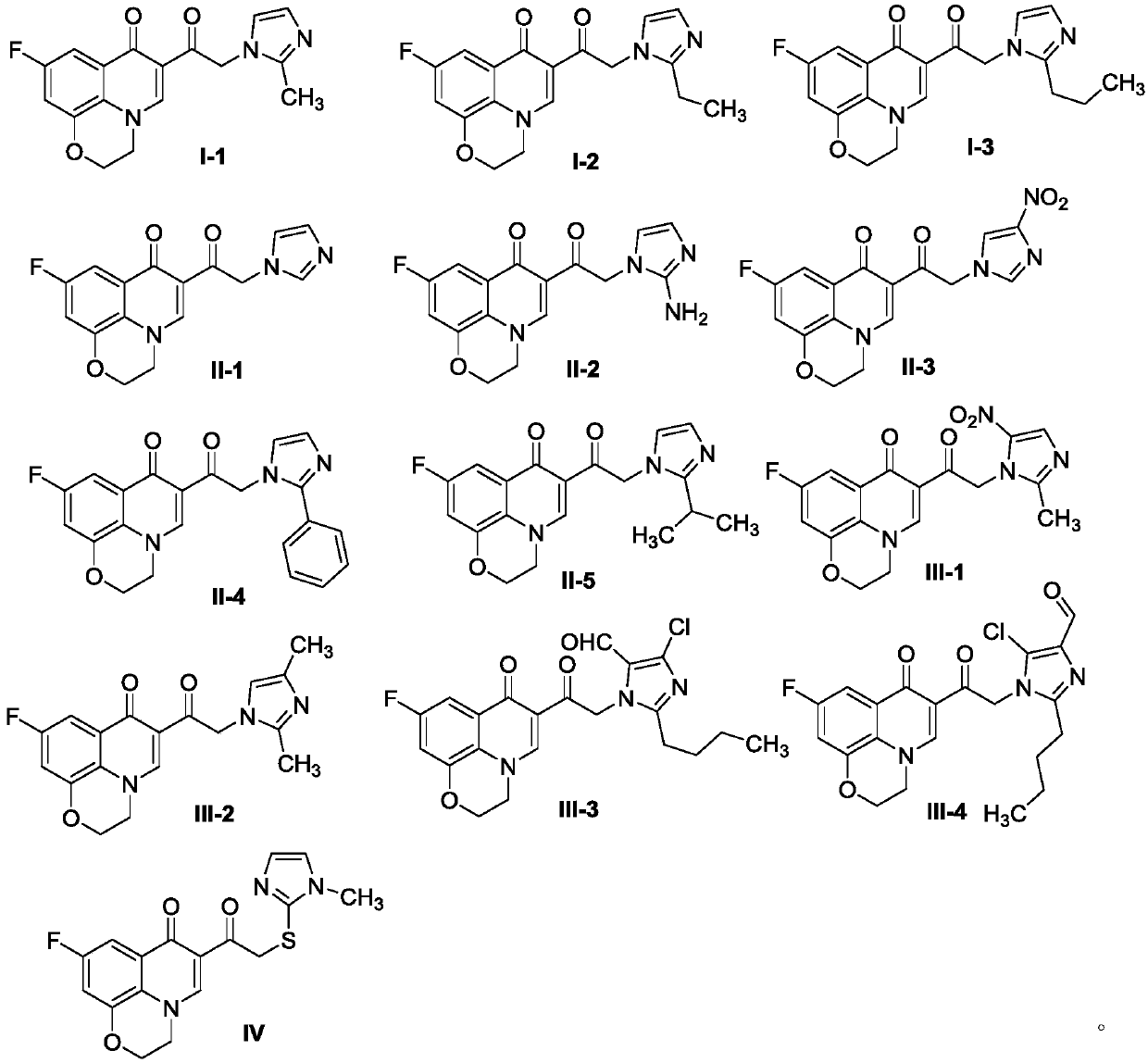
Quinolone imidazole compound as well as preparation method and application thereof
A technology of quinolone imidazoles and compounds, which is applied in the field of quinolone imidazoles and their preparations, can solve the problems that traditional medicines cannot exert high-efficiency effects, and achieve the effects of solving drug resistance, short synthesis route, and simple preparation of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
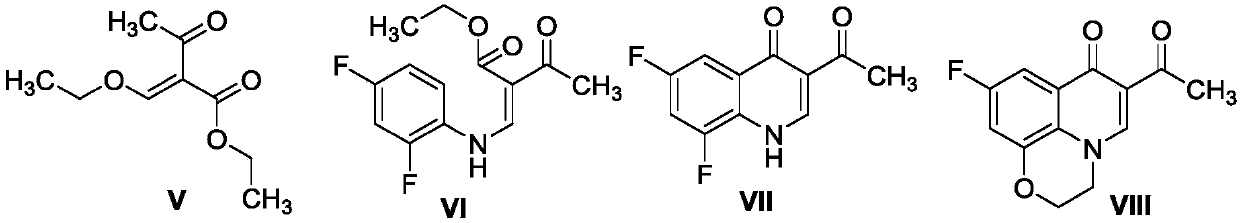
[0041] Preparation of Intermediates V-VII
[0042]
[0043] Reference "Cui Sheng-Feng, Addla Dinesh, Zhou Cheng-He. Novel 3-Aminothiazolquinolones: Design, Synthesis, Bioactive Evaluation, SARs, and Preliminary Antibacterial Mechanism. Journal of Medicinal Chemistry, 2016, 59, 4488-4510" disclosed method for preparation. Obtained 2.45g of intermediate V with a yield of 70.1%, black oily liquid; obtained 2.59g of intermediate VI with a yield of 76.3% as a brown solid; obtained 3.05g of intermediate VII with a yield of 52.8% as a brown solid.
Embodiment 2
[0045] Preparation of Intermediate VIII
[0046]
[0047] In a 250mL round bottom flask, add intermediate VII (1g, 4.48mmol) and potassium carbonate (1.86g, 13.45mmol) in 25mL of N,N-dimethylformamide at 100°C for 1h, then add bromoethanol (0.35 mL, 4.93mmol), stirred at 100°C for 24h. Thin-layer chromatography tracked to the end of the reaction, cooled to room temperature (18-25°C), and then concentrated, filtered, column chromatographically separated, dried and other post-treatments to obtain 1.10g of compound VIII, with a yield of 10.84% and a melting point of 242°C , brown solid. 1 H NMR (600MHz, DMSO-d 6 )δ8.53 (s, quinolone-5-H, 1H), 7.51–7.44 (m, quinolone-10-H, 1H), 7.32–7.26 (m, quinolone-8-H, 1H), 4.54 (d, J=4.2Hz, C2-OCH 2 ,2H),4.45(d,J=4.0Hz,C3-NCH 2 ,2H),2.61(s,-CH 3 ,3H).
Embodiment 3
[0049]Preparation of Intermediate IX:
[0050]
[0051] Intermediate VIII (0.40 g, 1.62 mmol) was stirred in acetic acid (10 mL) at 0 °C, bromine (0.13 g, 2.43 mmol) was added to the reaction mixture, and then stirred at 60 °C for 12 h. After the reaction was complete, the reaction mixture was cooled to room temperature, diluted with 25 mL of water, and the resulting solid was filtered and washed twice with water to remove acetic acid. After concentration, separation by column chromatography, drying and other post-treatments, intermediate IX was obtained with a yield of 28.6%, a melting point of 240° C., and a brown solid. 1 H NMR (600MHz, DMSO-d 6 )δ8.67(s,1H,quinolone-5-H),7.50(dd,J=9.1,2.0Hz,1H,quinolone-10-H),7.33(dd,J=9.4,2.2Hz,1H,quinolone -8-H),4.91(s,2H,CH 2 Br), 4.58–4.55 (m, 2H, OCH 2 ),4.52–4.46(m,2H,NCH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com