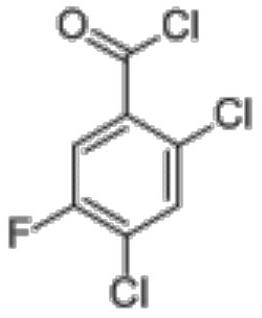
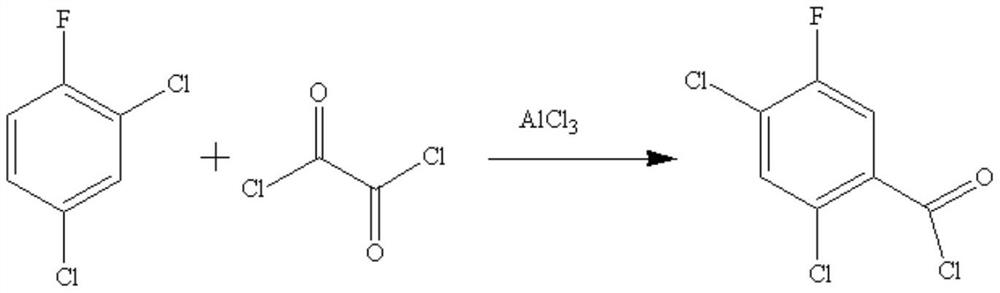
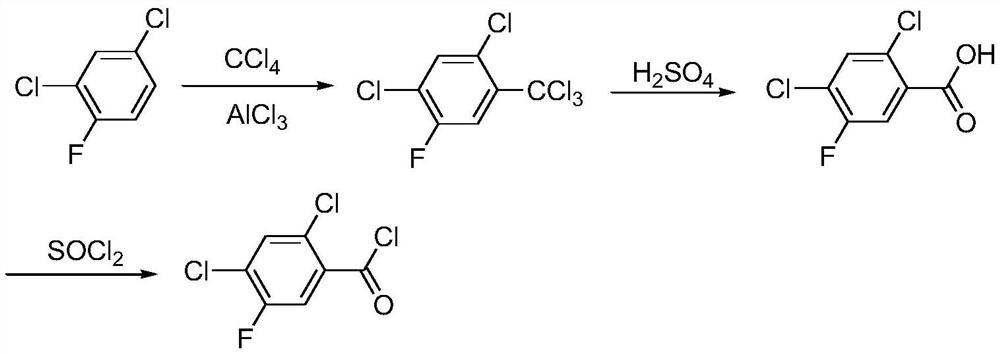
A kind of synthetic method of 2,4-dichloro-5-fluorobenzoyl chloride
A technology for the synthesis of fluorobenzoyl chloride and its synthesis method, which is applied in the field of synthesis of fine chemical intermediates, which can solve the problems of the use of highly toxic chemicals, excessively long process routes, and high equipment requirements, and achieve less pollutant discharge, short process routes, and high production efficiency. good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0066] Treat the cationic resin with 1mol / L sodium hydroxide first, then wash it with hydrochloric acid, wash it with water until it is neutral, and then immerse it in 0.5mol / L FeCl 3 Stir in the solution for 8 hours, then slowly add HF until the pH is 2 to 3; age the reaction system at -5°C for 24 hours, filter and wash with suction, then dry the filter cake at 80°C to obtain a cationic resin Catalyst (ie catalyst Cat.1).
Embodiment 2-1
[0069] Add 165g (1mol) of 2,4-dichlorofluorobenzene and 0.77g of Cat. 2 to 2MPa, stirred and reacted for 3 hours to obtain a reaction solution; then add CCl dropwise to the reaction solution 4 15.4g (0.1mol), heat up to 60°C, keep warm for 2 hours, the hydrogen chloride generated by the reaction is absorbed by falling film, after the reaction, the material obtained by the reaction is filtered to recover the catalyst Cat.1, and the filtrate is distilled under reduced pressure to obtain 2 , 44.95g of 4-dichloro-5-fluorobenzoyl chloride, yield 98.8%.
Embodiment 2-2
[0071] Add 330g (2mol) of 2,4-dichlorofluorobenzene and 1.54g of catalyst Cat.1 into the reaction flask, first raise the temperature to 40°C, and then introduce 2 to 2MPa, stirred and reacted for 4 hours to obtain a reaction solution; then add CCl dropwise to the reaction solution 4 15.4g (0.1mol), heat up to 60°C, keep warm for 2 hours, the hydrogen chloride generated by the reaction is absorbed by falling film, after the reaction, the material obtained by the reaction is filtered to recover the catalyst Cat.1, and the filtrate is distilled under reduced pressure to obtain 2 , 44.8g of 4-dichloro-5-fluorobenzoyl chloride, yield 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com