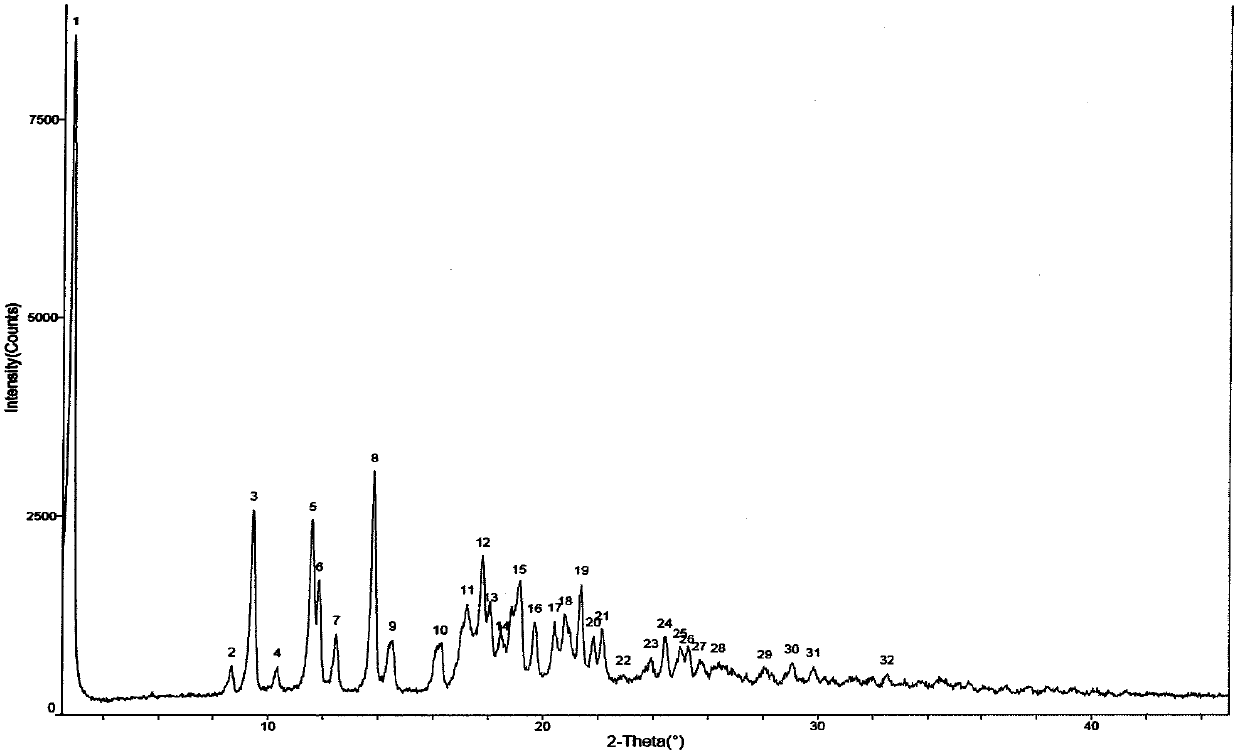
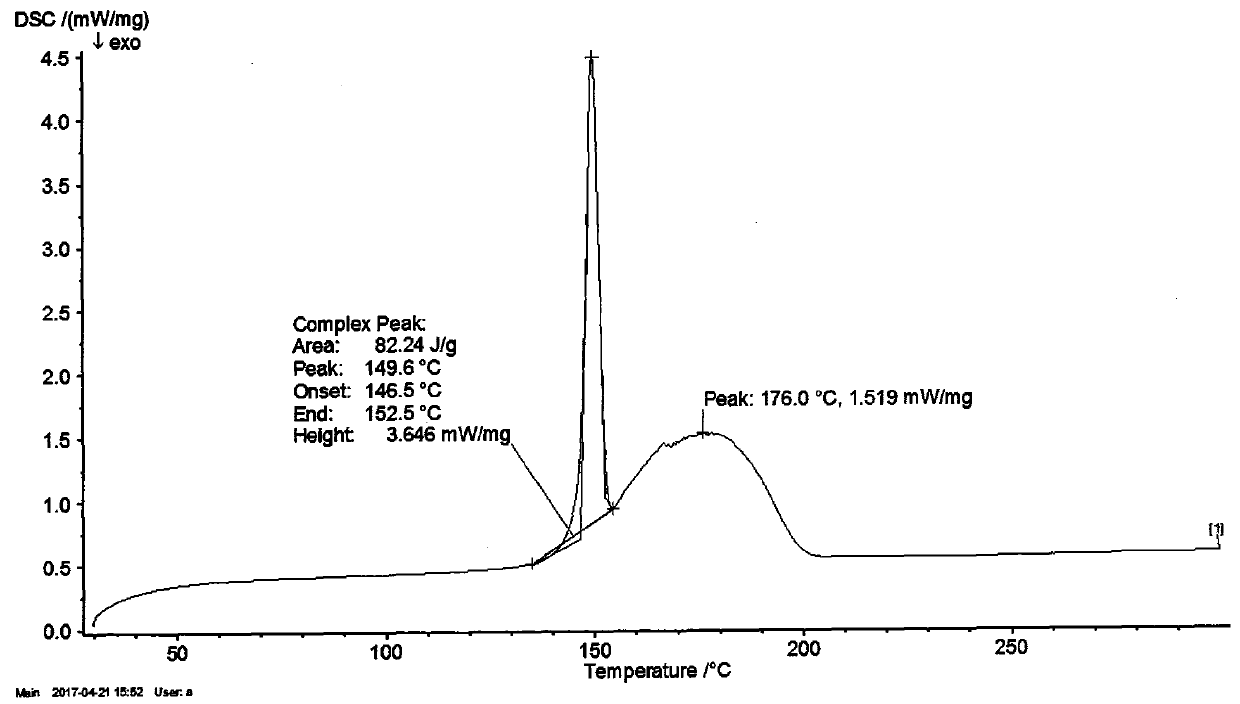
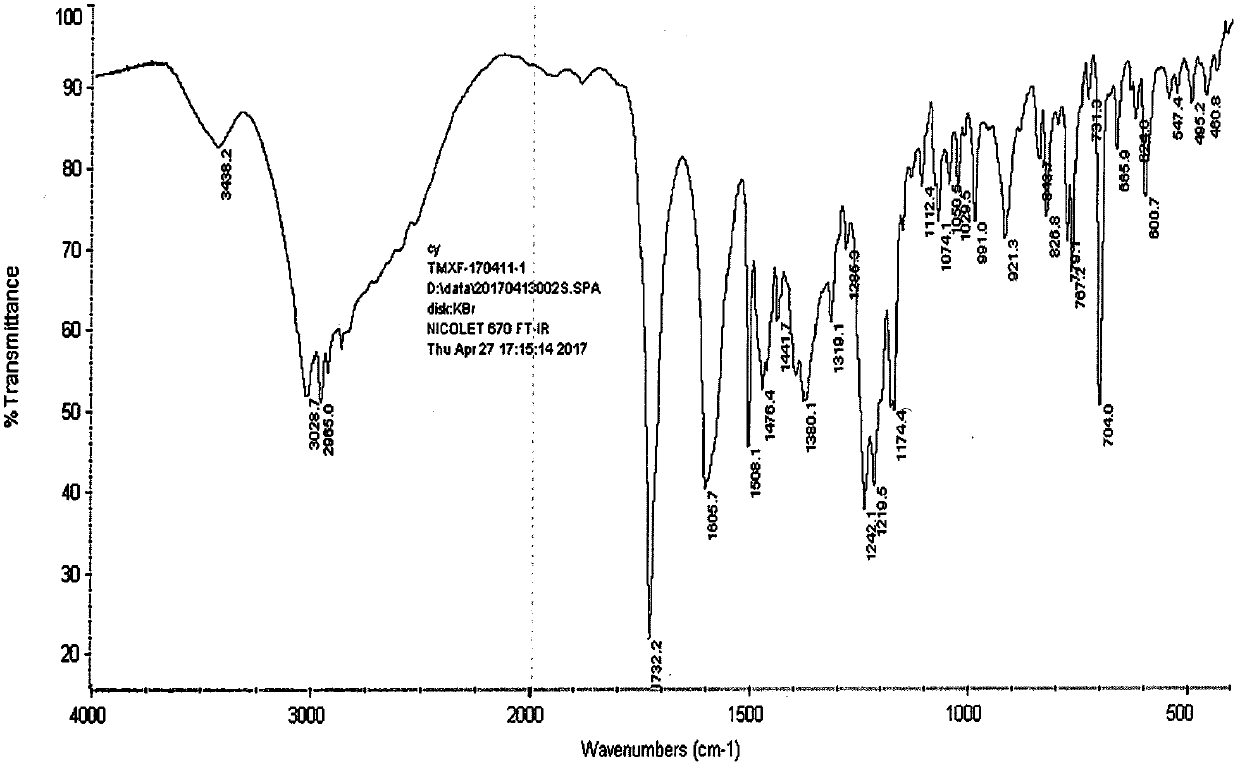
Industrialization method for preparing tamoxifen citrate polycrystalline type A
A citric acid and polymorphic technology, applied in the field of medicinal chemistry, can solve problems such as difficult realization, increased impurities, and difficult industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1 tamoxifen citrate crystal form A:
[0046] Add 10.00 g of the crystal form of tamoxifen citrate into DMF (20ml), heat to dissolve at 60°C, and then slowly add it to xylene (200ml) pre-cooled at -20°C, at -10±5°C, After continuing to stir for 2-3 hours, filter, wash with a small amount of DMF:xylene (1:10), and dry at 60°C to obtain about 9.41g of polymorph A of tamoxifen citrate, mp: 143-145°C, HPLC 99.85%, maximum simple 0.05%, HPLC: 99.72%.
Embodiment 2
[0047] The preparation of embodiment 2 tamoxifen citrate crystal form A:
[0048] Add 10.00g of tamoxifen citrate to DMF (15ml), heat to dissolve at 60°C, then add it to pre-cooled toluene (200ml) with stirring, continue to stir at -10±5°C for 2 hours, then filter , washed with a small amount of DMF:toluene (1:10), and dried at 60°C to obtain about 9.28g of polymorph A of tamoxifen citrate, mp: 143-145°C.
Embodiment 3
[0049] The preparation of embodiment 3 tamoxifen citrate crystal form A:
[0050] Add 10.00g of tamoxifen citrate methanol solvate into DMF (15ml), heat to dissolve at 60°C, then add it to pre-cooled xylene (200ml) with stirring, and continue stirring at -10±5°C After 2 hours, filter, wash with a small amount of xylene, and dry at 60°C to obtain about 9.05 g of polymorph A of tamoxifen citrate, mp: 143-145°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com